Wireless Control of Redox Gradients in Conductive Hydrogels: A New Frontier in Drug Delivery & Energy Harvesting
Published in Bioengineering & Biotechnology, Chemistry, and Materials
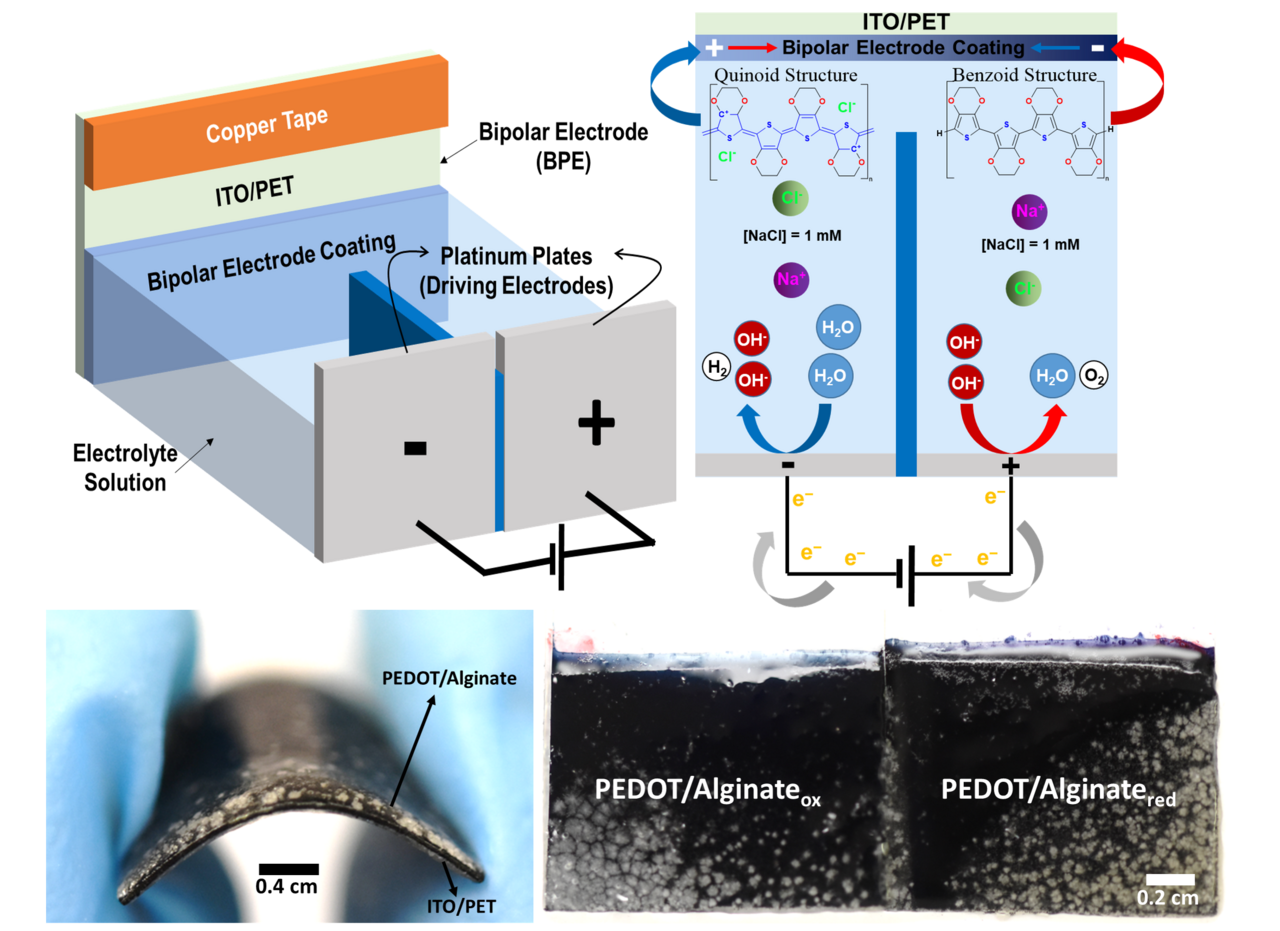
Bipolar electrochemistry enables the wireless modulation of redox states across conductive materials, offering new possibilities for localized electrochemical transformations without the need for direct electrical connections. By leveraging this principle, we developed a system where redox gradients can be precisely controlled, opening pathways for novel applications in bioelectronics and materials science. Our study demonstrates how BE can be harnessed to manipulate the chemical and physical properties of conductive polymer-hydrogel hybrids, paving the way for innovative functional materials with tunable characteristics.
We are excited to share our latest publication exploring bipolar electrochemistry (BE) as a wireless strategy to spatially control redox gradients in conductive polymer-hydrogel hybrids (Figure 1)!
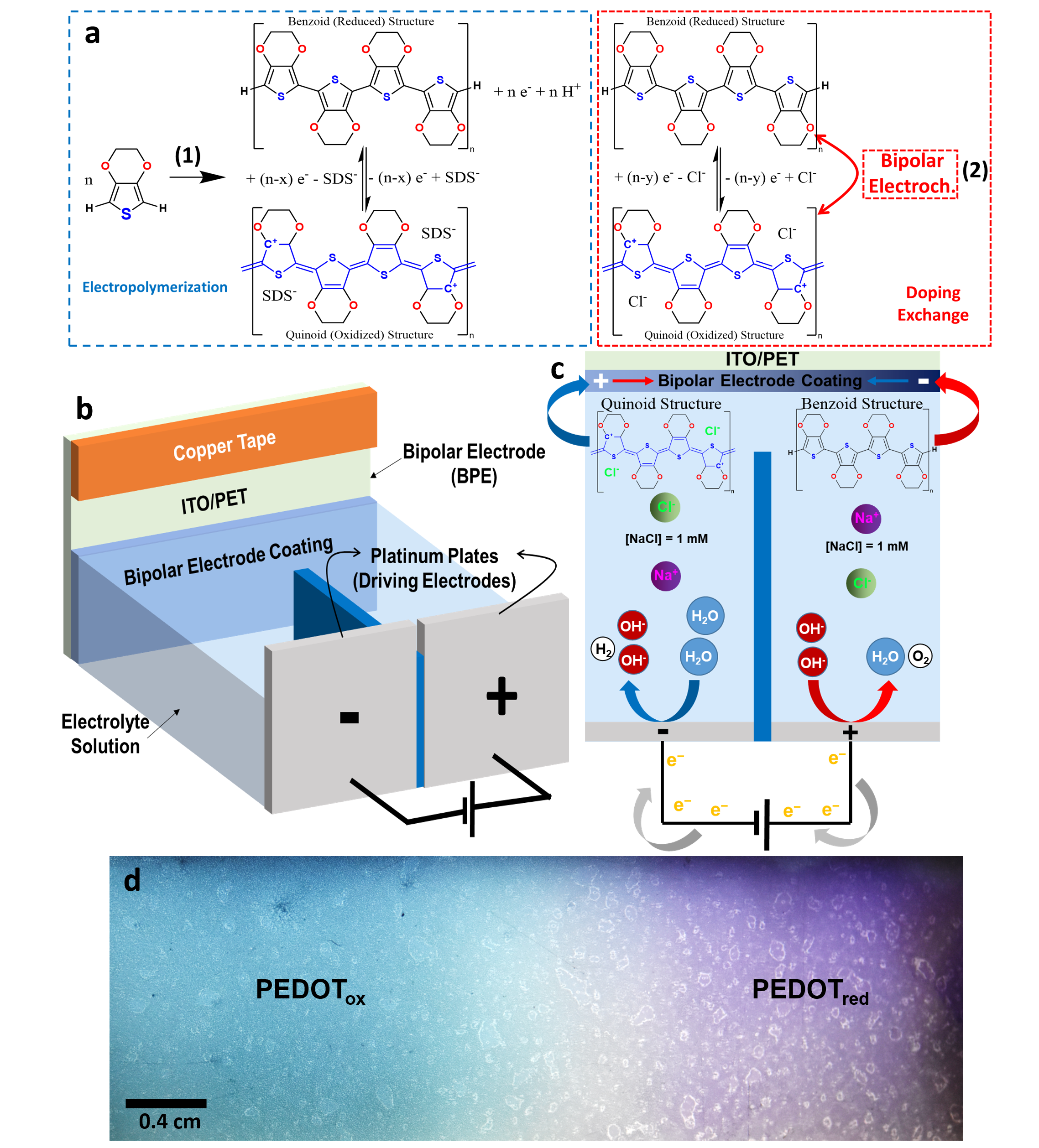
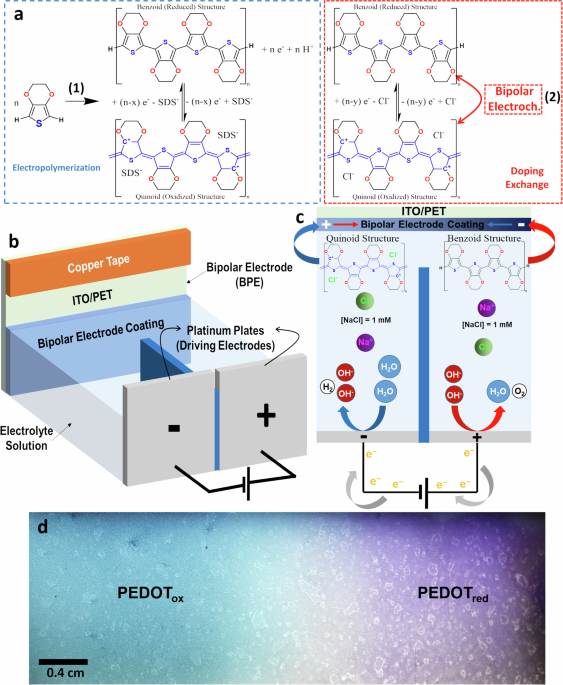
By integrating poly(3,4-ethylenedioxythiophene) (PEDOT) with alginate hydrogels, we developed flexible bipolar electrodes (BPEs) capable of wireless and reversible redox tuning (Figure 2). This unique combination of a conductive polymer and biocompatible hydrogel matrix provides a mechanically adaptable platform for dynamic redox control. (10.20517/ss.2022.25; 10.1002/mame.202300263) The wireless nature of the system allows for seamless integration into biomedical and electronic devices, facilitating localized electrochemical processes in a completely untethered manner. This approach holds great promise for applications requiring spatially controlled chemical reactions, from responsive biointerfaces to advanced energy storage solutions.
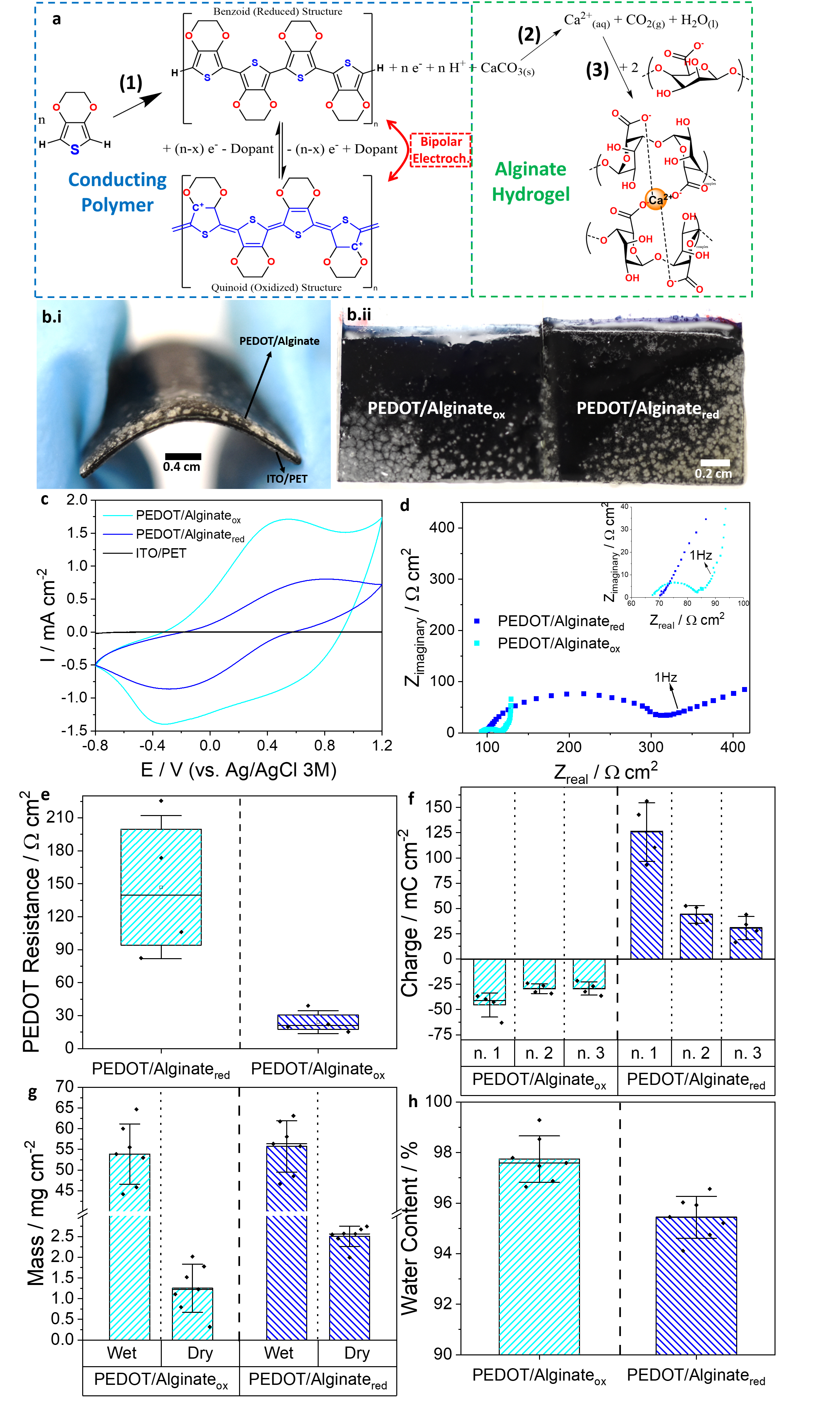
PEDOT (benzoid structure) and oxidized PEDOT (quinoid structure, represented in blue). PEDOT electropolymerization takes place in parallel with (2) decomposition of calcium carbonate, and (3) complexation with alginate macromolecules,
resulting in formation of the alginate hydrogel (green dashed square). The redox equilibrium of PEDOT can be subsequently controlled by bipolar (wireless) electrochemistry. b Pictures of: (i) the entire BPE subjected to bending, and (ii) the oxidized (left) and reduced (right) sides following BE activation. c The 10th cycle of CV for the bare ITO/ PET (black), PEDOT/Alginateox (light blue) and PEDOT/Alginatered (dark blue), recorded in PBS at 50 mVs−1. d Nyquist plot of EIS for the PEDOT/Alginateox (light blue) and PEDOT/Alginatered (dark blue), recorded in PBS. e PEDOT resistance obtained from EIS for oxidized (right) and reduced (left) halves. f Charge storage capacity of reduced and oxidized BPE halves was obtained by applying +1.2 V or −0.8V (vs Ag/AgCl/KCl 3M) respectively for 30 min while measuring the resulting currents. Later, the BPEs were electronically reconnected using a new copper tape and placed back in the bipolar electrochemical cell as BPE electrode for a new cycle (n. 1, 2 and 3). g Wet mass(left) and dry (right) mass of hydrogels in the oxidized (light blue)
and reduced (dark blue) halves of the BPE. h Water content in the oxidized (light blue) and reduced (dark blue) halves of the BPE. Error bars in (e–h) are standard deviations (see “Methods”).
🔹 Targeted drug loading – Fluorescein molecules were selectively loaded into specific regions of the hydrogel, demonstrating an alternative to conventional uniform doping techniques (Figure 3). This technique enables spatial control over the incorporation of bioactive molecules, allowing for the development of next-generation drug delivery systems. By leveraging BE, we demonstrate how wireless modulation can be used to create patterned drug reservoirs, which can be released in a controlled manner. This advancement is particularly relevant for precision medicine, where localized and programmable drug release is critical for improving therapeutic outcomes.
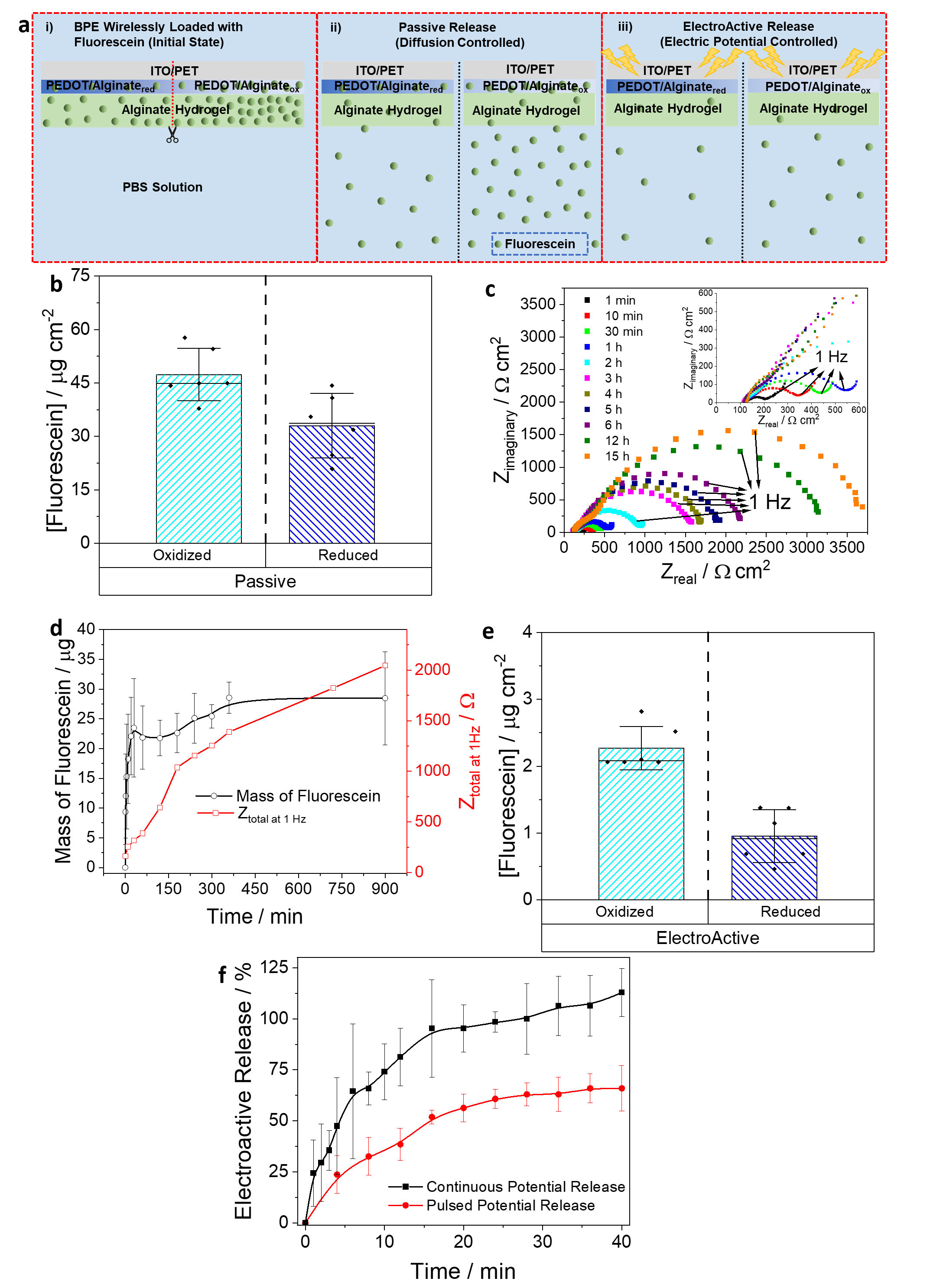
🔹 Energy harvesting – By cutting gradient-encoded BPEs and closing an external circuit, we recovered stored electrochemical energy using a concentration cell mechanism (Figure 4). This innovative approach transforms our conductive hydrogel system into a wireless energy storage platform, capable of harnessing and releasing stored charge based on redox potential differences. The ability to wirelessly charge and discharge these materials suggests potential applications in self-powered biosensors, flexible electronics, and even implantable medical devices requiring localized power sources.
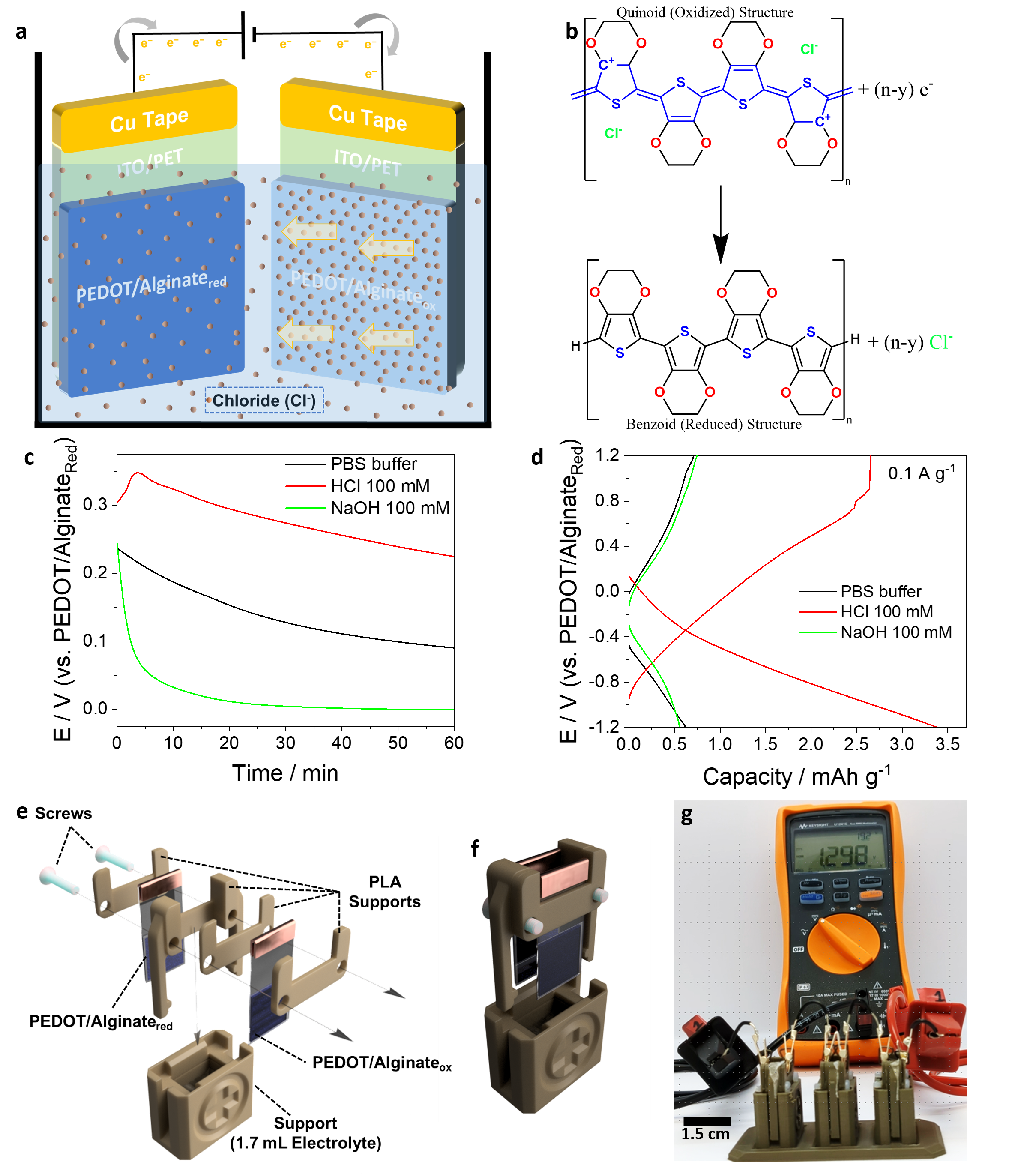
PEDOT/Alginateox. c Electrical potential generated over time for different electrolyte solutions: PBS buffer (black), 100 mM hydrochloric acid (red), and 100 mM sodium hydroxide (green). d Galvanostatic charge-discharge curves for the different electrolytes at 0.1 A g−1. e Schematic representation of the single-cell design using 3D-printed parts. f Schematic representation of an assembled single-cell. g Photograph of three single-cell batteries connected in series to a multimeter measuring the generated DC voltage.
Using cyclic voltammetry, impedance spectroscopy, Raman microscopy, and XPS, we mapped distinct redox regions within the BPEs, providing insights into wireless bioelectronics applications. (10.1038/s41467-022-29037-6)
This work highlights the untapped potential of BE in biomedical and energy fields, bridging materials science, electrochemistry, and bioengineering for next-generation drug delivery and energy storage systems.
Looking forward to discussions with fellow researchers on how BE can revolutionize wireless chemical control in functional materials!
Read more: https://www.nature.com/articles/s43246-025-00750-1 / Bipolar electrochemistry-driven wireless drug loading and energy harvesting in conductive hybrid hydrogels | Communications Materials
#BipolarElectrochemistry #Bioelectronics #Hydrogels #DrugDelivery #EnergyHarvesting #MaterialsScience #Electrochemistry
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
-
Communications Materials

A selective open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of materials science.
Your space to connect: The Fuel cell technologies Hub
A new Communities’ space to connect, collaborate, and explore research on Electrochemistry, Chemical Engineering, and Fuel Cells!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Materials for quantum sensing and computing
Publishing Model: Open Access
Deadline: Apr 09, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in