Electrochemical CO2 Reduction to Methane by Triazole Molecules
Published in Chemistry
Renewable-electricity triggered electrochemical CO2 reduction reaction (CO2RR) is a promising opportunity to produce carbon-neutral fuels and chemicals. Hydrocarbons and oxygenate products with high energy density, such as CH4, C2H4 and C2H5OH, are especially pursued. Developing catalysts with high selectivity and activity for this reaction is crucial to lower the downstream separation cost.
Previous research has shown that Cu is the most effective catalyst for producing hydrocarbons and oxygenates, but it suffers from poor intrinsic selectivity — yielding up to 16 products. The diverse binding sites on the Cu surface lead to a wide array of intermediates in CO2RR that produce varied products. Molecules with well-defined structures and narrower “electronic states” (orbitals) could be promising. Although some organic molecules have been reported to catalyse CO2RR, the overall activity is limited, raising the debate on product identification. Thus, this calls for organic molecular catalysts that can improve selectivity and stability under high current conditions.
Our promising candidate is 3,5-diamino-1,2,4-triazole (DAT), which can activate CO2 due to its favourable electronic properties. In-situ Raman spectroscopy confirmed the activation of CO2 by DAT under applied potential. We developed DAT-based gas diffusion electrodes to increase the concentration of active sites, resulting in enhanced performance in membrane electrode assembly (MEA) electrolysers (Figure 1a). The DAT electrode (electrode area 4 cm2) exhibits a high Faradaic efficiency (FECH4) of 52% for CO2-to-CH4 under 250 mA cm-2 in MEA (Figure 1b). Although traces of metal impurities were detected in the electrode — an unavoidable aspect of device fabrication — DAT proved crucial for the CO2-to-CH4 conversion process. Experiments using carbon monoxide (CO) as a feed gas and formaldehyde (HCHO) revealed deactivation of the electrode for CO2RR, suggesting an alternative reaction pathway distinct from the commonly accepted *CO intermediates. With the aid of DFT calculation, we suggest a possible *CO2-*COOH-*C(OH)2-*COH pathway to produce CH4 on DAT (Figure 1c).
We successfully scaled up the DAT electrode from 4 cm2 to 81 cm2 in MEA, where the 81 cm2 DAT electrode delivers a FECH4 of 54% at a total current of 10 A, together with a CH4 production rate of 23 mmol h-1 (Figure 1d). By adjusting the CO2 flow rate, the 81 cm2 DAT electrode can directly produce town gas from CO2 (Figure 1e), underscoring the potential of molecular electrodes for real-world applications.
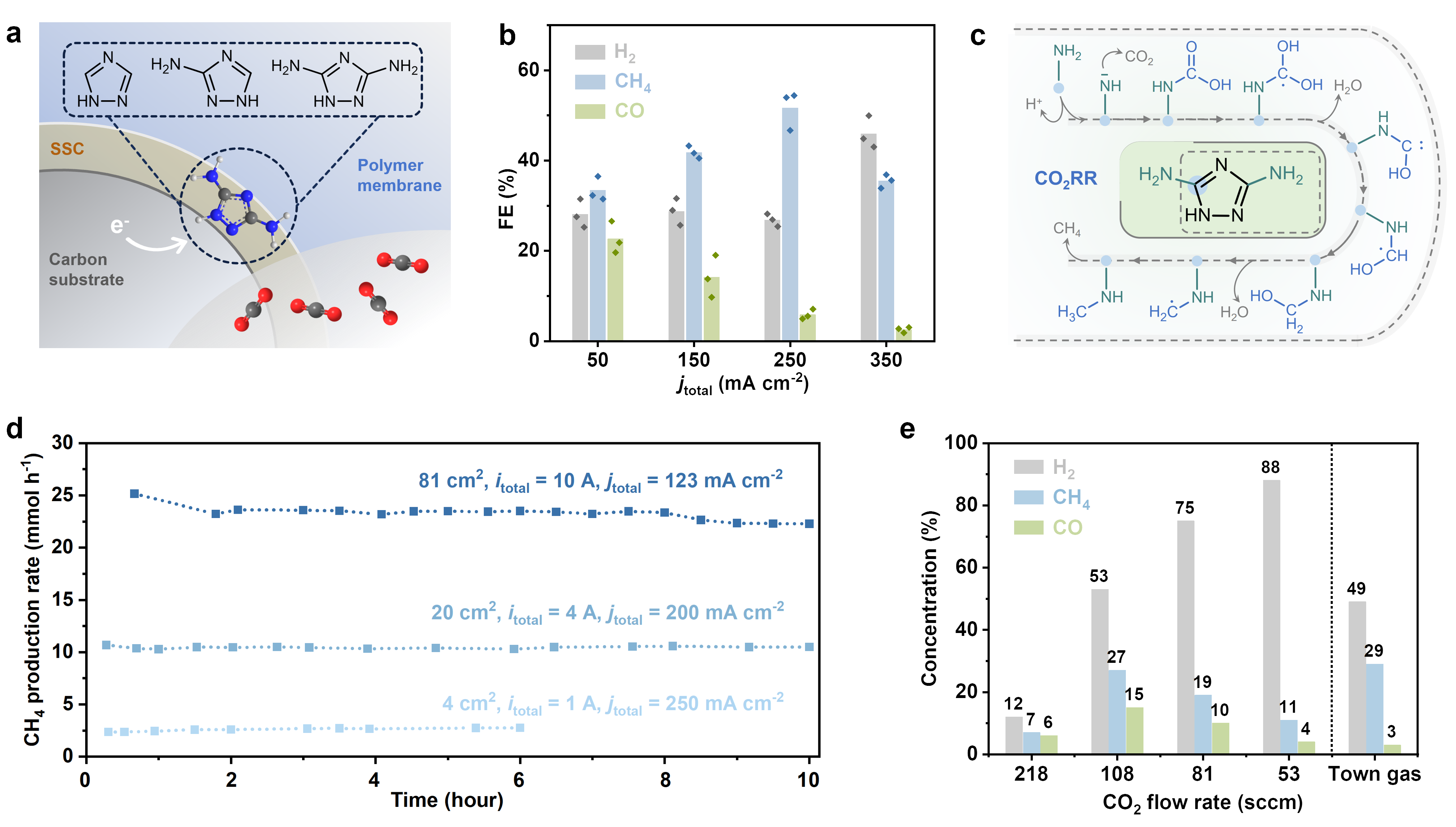
Figure 1. (a) Schematic of molecular catalysts on electrode for CO2RR in MEA. (b) The FE of major products from CO2RR at different current densities from 50 to 350 mA cm-2 on DAT electrodes. The values of bar chart are means. (c) Proposed CO2RR pathway on DAT. (d) The production rate of CO2-to-CH4 on DAT electrode with an effective area of 4, 20 and 81 cm2. (e) Concentration of gas products on 81 cm2 DAT electrode under total current of 10 A (current density = 123 mA cm-2) with different flow rate of CO2.
Follow the Topic
-
Nature Energy

Publishing monthly, this journal is dedicated to exploring all aspects of this on-going discussion, from the generation and storage of energy, to its distribution and management, the needs and demands of the different actors, and the impacts that energy technologies and policies have on societies.
Related Collections
With Collections, you can get published faster and increase your visibility.
Microgrids and Distributed Energy Systems
Publishing Model: Hybrid
Deadline: Mar 31, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
Well, triazole was synthesized with copper catalysts and copper single site was known to produce methane.