Figuring out a Matryoshka
Published in Chemistry
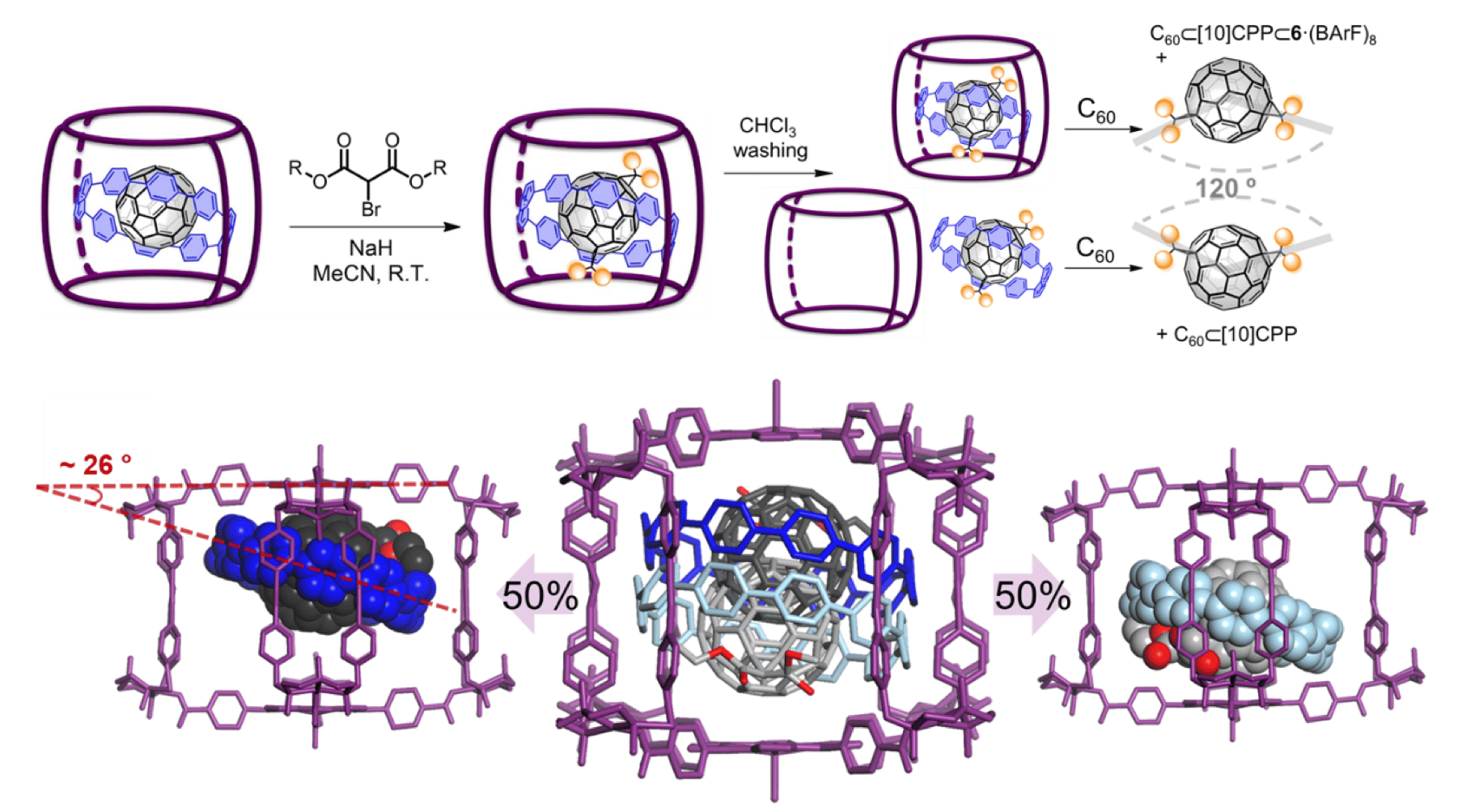
Our QBIS-CAT research group has been working on supramolecular nanocapsules for nearly a decade. In 2014 a tetragonal prismatic nanocapsule capable of tightly encapsulating fullerenes from C60 to C84, featuring a large cavity formed after the self-assembly of Zn-porphyrins and a macrocyclic Pd-based complex.1 This was the beginning of a successful story along these years on the study of chemistry at the confined space of the nanocapsules, from catalyst encapsulation and enantioselective catalysis2,3 to fullerene and endohedral metallofullerene (EMF) purification.1,4 More recently, the supramolecular nanocages were used as masks for the regioselective formation of tetrakis-adducts at the equatorial belt, matching the 4-gate cross-shaped geometry of the nanocapsule.5 This was a milestone in the field and prompted us to continue the investigation of the supramolecular mask strategy.
It was in Summer 2018, during the ISMSC conference in Quebec, that the idea to merge the supramolecular masks with the work from the group of Dr. Max von Delius popped up, after staring at their poster on the Bingel functionalization of C60 being trapped in the cycloparaphenylene [10]CPP.6 It was there when Carles Fuertes and Xavi Ribas met Max for the first time and discussed about the possible strategy to encapsulate C60Ì[10]CPP in a larger nanocapsule and explore the a putative highly selective functionalization of C60 taking advantage of both the supramolecular mask and the cycloparaphenylene. Thus, taking advantage to the facile modulation of the distance between porphyrins by changing the macrocyclic complex structure, in this work, we developed a larger nanocapsule to host C60Ì[10]CPP, thus featuring a ternary complex with a Matryoshka-like resemblance that was thoroughly characterized via dozens of host-guest titrations, carried out by Oleg Borodin from the Delius group. Accordingly, we submitted this new three-shell complex to Bingel cyclopropanation conditions to realize that it was an exquisite system to show complete regioselectivity for trans-3 bis-adducts of C60. After several attempts of crystallization, good single crystals of both the unreacted Matryoshka and the trans-3 bis-adduct-Matryoshka were obtained and we succeed in their diffraction at ALBA Synchrotron in Barcelona, thanks to the ongoing collaboration with Prof. Maspoch’s group. The crystallographic data was crucial to understand the precise reactivity at two specific [6,6] bonds of the C60sphere, giving the trans-3 fullerene bis-adduct. The fact that the cycloparaphenylene ring has a significant inclination with respect the porphyrin plane plays an important role in the site-selective reactivity to the fullerene.
At this point, we decided to inspect other bromomalonates to screen the impact of their bulkiness on the reaction outcome. Compared to the good yields obtained for diethyl bromomalonate, the bulkier diisopropyl and dibenzyl bromomalonates showed a gradually decreasing yield, indicating that the steric hindrance blocks the accessibility to the trans-3 [6,6] bonds. The diminished reactivity is nicely correlated to the Rebek’s rule for 55% maximum occupancy of the cavity, since the bulkier bromomalonates are at the limit or surpass the 55% occupancy.
The understanding on how to control the regioselective functionalization of fullerenes gained with this new matryoshka-like supramolecular system may lead to the implementation of the fullerenes derivatives in a wide range of electronic devices and solar cells. We might expect new exciting results in the supramolecular regiofunctionalization of fullerenes in the upcoming years considering there is a lot more to explore, from variation of the nature of the fullerene guest, modulation of the nanocapsule structure, or investigating new reaction patterns. Check out more about our recent work at: https://dx.doi.org/10.1038/s41557-021-00658-6 .
Ernest Ubasart & Xavi Ribas
- García-Simón, C.; García-Borràs, M.; Gómez, L.; Parella, T.; Osuna, S.; Juanhuxi, J.; Imaz, I.; Maspoch, D.; Costas, M. & Ribas, X. Nat. Commun. 2014, 5, 5557-5565.
- García-Simón, C.; Gramage-Doria, R.; Raoufmoghaddam, S.; Parella, T.; Costas, M. Ribas, X & Reek, J. N. H. J. Am. Chem. Soc. 2015, 137, 2680-2687.
- Colomban, C.; Martín-Diaconescu, V.; Parella, T.; Goeb, S.; García-Simón, C.; Lloret-Fillol, J.; Costas, M. & Ribas, X. Inorg. Chem. 2018, 57, 3529-3539.
- Fuertes-Espinosa, C.; Gómez-Torres, A.; Morales-Martínez, R.; Rodríguez-Fortea, A.; García-Simón, C.; Gándara, F.; Imaz, I.; Juanhuix, J.; Maspoch, D.; Poblet, J. M.; Echegoyen, L. & Ribas, X. Angew. Chem. Int. Ed. 2018, 57, 11294-11299.
- Fuertes-Espinosa, C.; García-Simón, C.; Pujals, M.; García-Borràs, M.; Gómez, L.; Parella, T.; Juanhuix, J.; Imaz, I.; Maspoch, D.; Costas, M. & Ribas, X. Chem. 2020, 6, 169-186.
- Xu, Y., Kaur, R., Wang, B., Minameyer, M.B., Gsänger, S., Meyer, B., Drewello, T., Guldi, D.M., and von Delius, M. J. Am. Chem. Soc. 2018, 140, 13413–13420.
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in