High-performance piezoelectric/ferroelectric bio-organic films for sensor, energy harvester, and biomedical applications
Published in Materials

Although people are endeavoring to develop synthetic piezoelectric materials, nature seems to have grasped the effect for millions of years.
In 1880, Curie brothers first discovered and demonstrated the piezoelectric effect, which is an intrinsic property of crystals with a non-centrosymmetric structure that allows robust and precise conversion between electrical and mechanical energies. Indeed, the piezoelectric effect has been observed in diverse biological systems. From the first discovery of piezoelectricity in wool and hair in 1941 to the Nobel Prize in Physiology or Medicine in 2021, which revealed that cells perceive pressure and trigger tactile sensation through the electromechanical coupling effect of proteins Piezo 1 and Piezo 2, piezoelectric biomaterials have always been mysterious and attractive to researchers. The generation of bioelectricity from the piezoelectric effect has physiological significance in living systems, such as the piezoelectric charges generated by the human tibia during walking, which affects bone remodeling and growth. Additionally, piezoelectric potential in the lung generated during respiration could assist in binding oxygen to hemoglobin.
Recently, piezoelectric biomaterials have garnered great interest in biomedical applications due to their biocompatibility, biodegradability, accessibility, and environmental sustainability. However, the piezoelectric response of biomaterials (bone:0.2 pm V-1) is very weak compared to piezoceramics (PZT: 500 pm V-1) and even polymers (PVDF: 30 pm V-1) because of their random polarization and lack of ferroelectricity. Besides, the large-scale assembly and domain alignment of piezoelectric biomaterials remain challenging. Among the biomaterials, glycine, the simplest non-chiral amino acid, has three distinct crystallization polymorphs, non-piezoelectric α-glycine, piezoelectric β-glycine, and piezoelectric γ-glycine. β-glycine crystals exhibit high shear piezoelectricity (178 pm V-1) and marvelous piezoelectric voltage coefficient (8 Vm N-1) larger than any currently used ceramic or polymer. Unfortunately, β-glycine is the most difficult to form in kinetics and the most unstable in thermodynamics under ambient conditions. The excessively high coercive electric field also makes it quite challenging to polarize glycine crystals and align the domains at the macroscale, even though they are ferroelectric.
In our work, we present an active self-assembly strategy to tailor piezoelectric biomaterial thin films via synergistic nanoconfinement and in-situ poling. The nanoconfinement-induced homogeneous nucleation overcomes the interfacial dependency and allows the electric field applied in-situ to align ferroelectric domains across the entire film during synthesis. The β-glycine films exhibit an enhanced piezoelectric strain coefficient of 11.2 pm V-1 and an exceptional piezoelectric voltage coefficient of 252×10-3 Vm N-1, which is an order of magnitude larger than that of the state-of-the-art PZT. Of particular significance is that the nanoconfinement effect greatly improves the thermostability before melting (192 °C).
The β-glycine nanocrystalline films are fabricated based on a bio-organic film printer using the electrohydrodynamic spray method (Fig. 1a). With the rapid water evaporation and the increasingly large surface-area-to-volume ratio of the nano-micro droplets, the glycine nucleus is formed in the β phase through the nanoconfinement effect (Figs. 1b, 1c, and 1d). The in-situ electric field in the crystal growth process induces the domain alignment of β-glycine nanocrystals, suggesting the net polarization direction [020] is parallel to the electric field (Fig. 1e). The as-synthesized film resembles the inorganic polycrystalline morphology and it can be constructed in variable sizes and customizable structures on various rigid or flexible substrates (Figs. 1f, 1g).
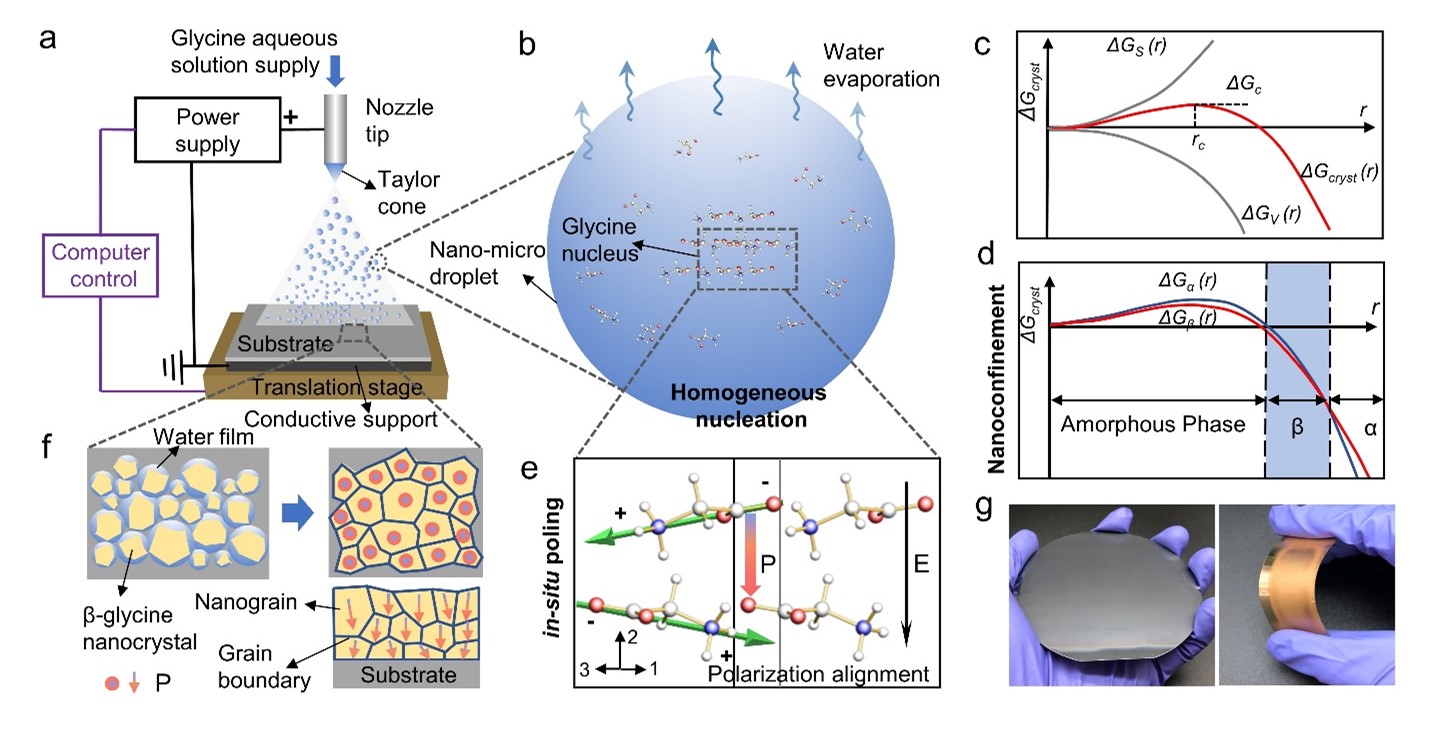
Fig. 1 Fabrication of piezoelectric β-glycine nanocrystalline films and the active self-assembly mechanism via synergistic nanoconfinement and in-situ poling. a, Schematic of the bio-organic films printer and the synthesis of β-glycine nanocrystalline films. b, Schematic of the nano-micro droplet of glycine solution and the crystallization process. c, Illustration of the free energy (ΔGcryst) profile of a growing crystal nucleus as a function of crystal radius, r. d, Illustration of the size-dependent free energy profiles for two competing nuclei corresponding to α-glycine and β-glycine. e, Schematic of orientation alignment of glycine molecules during homogeneous nucleation. f, Schematic of the film formation process showing the compact nanograins with uniform and consistent polarization orientation. g, Photographs of a film on a 4-inch silicon wafer (left) and film on a flexible gold-coated polyethylene terephthalate (PET) substrate (right).
The SEM images show the uniform and compact nanograin distribution of β-glycine films (Figs. 2a and 2b). XRD spectra confirm the pure β crystalline phase of the nanocrystalline films, where the strongest peak (020) manifests that the OOP orientation is dominated by being along the optimal piezoelectric direction (red curve in Fig. 2c). On the contrary, without either in-situ electric field or nanoconfinement, the β-glycine crystals show a dominant OOP orientation [001] that is perpendicular to the strongest piezoelectric direction (green curve and blue curve in Fig. 2c). They hardly exhibit piezoelectricity at a macroscopic level since the piezoelectric effects of the opposite domains will cancel out each other, which evidences the indispensable effect of the synergy of nanoconfinement and in-situ poling on domain alignment.
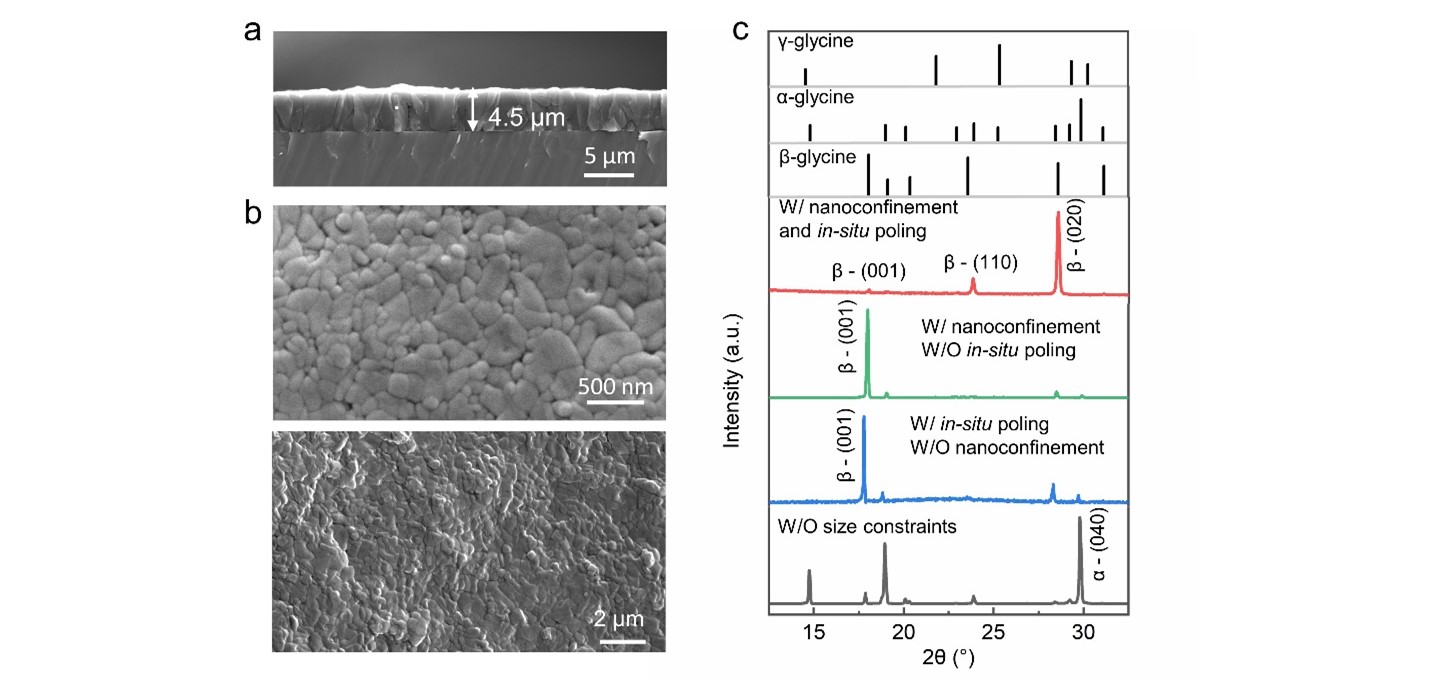
Fig. 2 Morphology and structural characterization of β-glycine nanocrystalline films. a, Cross-sectional SEM image of an as-obtained film with a thickness of 4.5 µm. b, Surface topography SEM image showing the compact nanosized grains of the uniform and continuous films. c, XRD spectra of the as-prepared β-glycine nanocrystalline films (red curve), β-glycine nanocrystals obtained in the absence of electric field (green curve), β-glycine microcrystals prepared by electrohydrodynamic focusing deposition (blue curve), and α crystals formed by direct evaporation of glycine solution film (black curve).
The piezoelectric properties of the as-prepared β-glycine nanocrystalline films are evaluated by piezoresponse force microscopy (PFM) measurements. Fig. 3a illustrates the PFM mapping of OOP amplitudes, exhibiting superb and uniform piezoelectric response of the compact nanocrystals. The PFM OOP phase mapping is uniform and exhibits hardly any opposite phase, indicating that the ferroelectric domains of the as-prepared films are well aligned, and the polarization direction of the nanograins is consistent (Fig. 3b). The linearly fitted effective piezoelectric coefficient is around 11.2 pm V-1 (red curve in Fig. 3c). which is superior compared to most reported bio-organic films (Fig. 3d). The uniform and high-value d33 could be attributed to the excellent alignment of ferroelectric domains due to in-situ poling during the β-glycine homogeneous nucleation, which exposes most of the (020) polar surface toward the OOP direction.
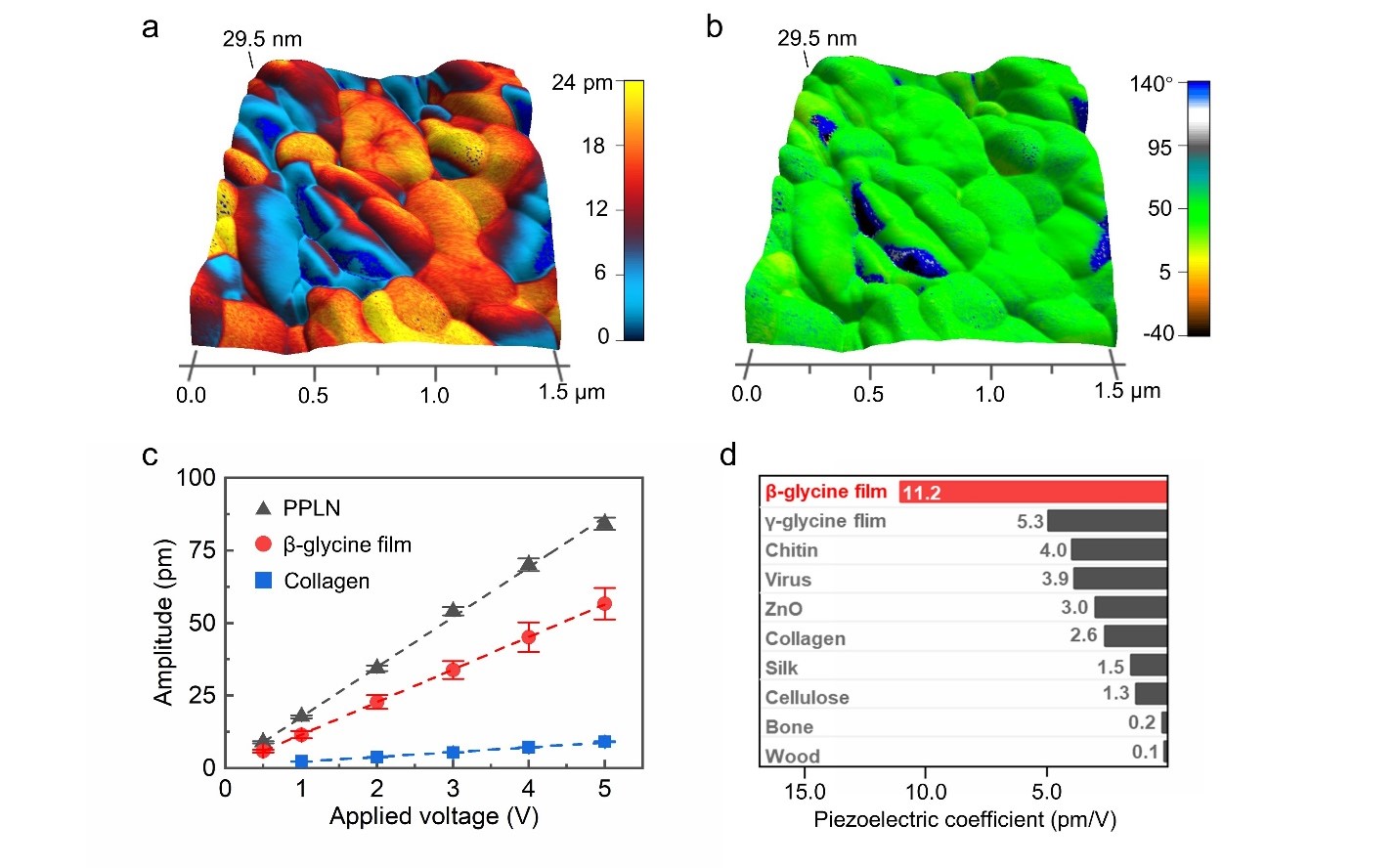
Fig. 3 PFM measurements and polarization alignment studies of β-glycine nanocrystalline films. a, The PFM OOP amplitude mapping overlaid on the 3D topography of as-prepared films. The applied AC voltage is 2 V. b, The corresponding PFM OOP phase mapping overlaid on the 3D topography. c, Linear dependence of PFM amplitude on the applied AC voltage. d, Piezoelectric coefficient of as-prepared β-glycine nanocrystalline films compared with other bio-organic piezoelectric materials.
Besides piezoelectricity, thermostability is also a key figure of merit of piezoelectric materials for applications over a broad temperature range. Unfortunately, bulk β-glycine crystals are the least stable and readily transform to α-glycine in moist air after being left at room temperature for several hours or heated to 67 °C. Hence, we investigate the thermostability and phase transition properties of the as-prepared β-glycine nanocrystalline films. Through the DSC and in situ XRD results, we confirm no phase other than the β phase is observed throughout the heating process, indicating the absence of a Curie transition prior to the melting temperature of 192 °C. The infinite thermostability of the films results from the nanoconfinement effect. This is supported by the Ostwald step rule, an otherwise metastable form of a crystalline substance, β-glycine, actually becomes the stable form when the crystal size is constrained to nanometer-scale dimensions. We then compare the piezoelectric voltage coefficient g33 of several actively studied piezoelectric material systems together with the β-glycine nanocrystalline films as a function of curie temperature (TC) (Fig. 4). The melting temperature of 192 °C of the β-glycine nanocrystalline films is higher than the TC of most piezoelectric materials and comparable to that of PZT-5H type piezoceramics. Notably, the g33 of the β-glycine nanocrystalline films is on the same order of magnitude as PVDF, but with much higher TC.
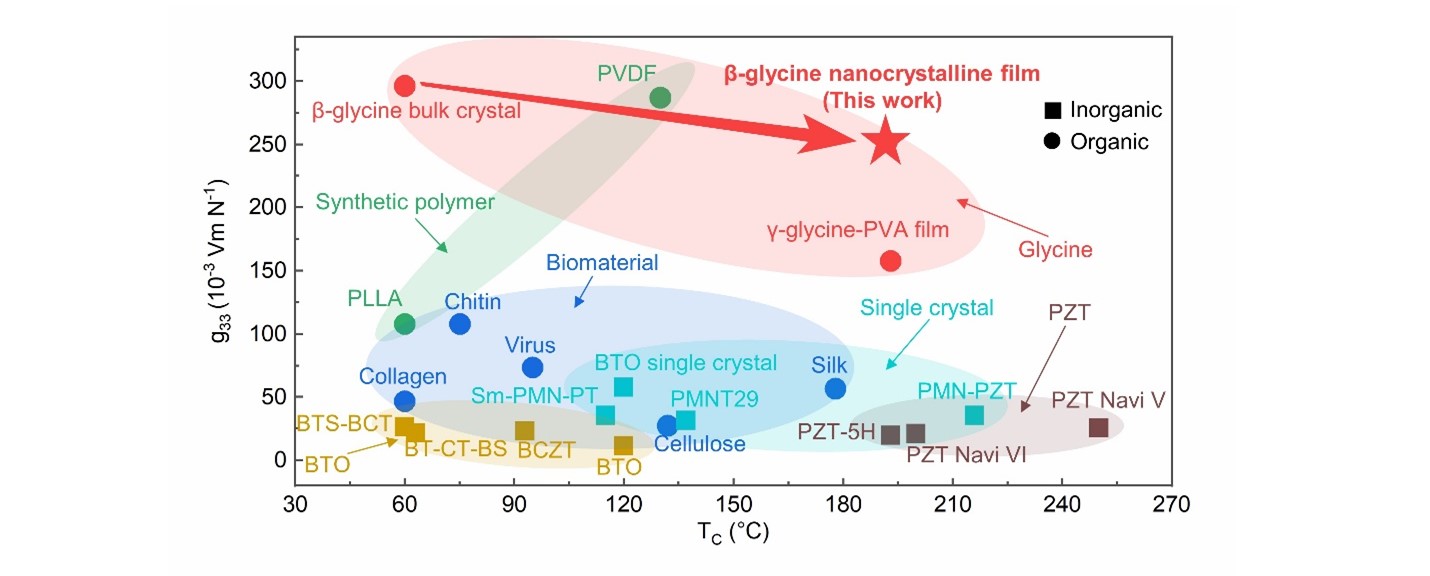
Fig. 4 Thermostability and overview of piezoelectric properties.
In summary, our work addresses the long-standing challenge of synthesizing large-scale high-performance piezoelectric biomaterials. The excellent output performance, natural biocompatibility, and biodegradability of the β-glycine nanocrystalline films are of practical implications for high-performance biological electromechanical microdevices and transient bioelectronics. The proposed strategy is scalable to create films with variable dimensions, programmable structures, and diverse material forms such as flexible composites. Furthermore, this finding offers a generally applicable strategy for designing large-scale films of various biomaterials and other piezoelectric materials such as molecular or organic-inorganic piezoelectric materials.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in