Researchers invent new resorbable biomaterials for implantable medical devices
Published in Materials

What if we had tiny device in our body that could constantly monitor damaged arteries, accelerate bone regeneration and wound healing, or facilitate the drug delivery for cancer treatment. This could open up remarkable opportunities for the treatment of human diseases and the enhancement of our capabilities beyond the limits of biology.
Piezoelectric biomaterials will be utilized to create these devices, which can generate electrical signals through the mechanical stress produced by bodily movements such as muscle stretching, breathing, blood flow, and small movements. These devices will not require batteries and will be designed to safely dissolve inside the body once they have fulfilled their purpose.
The 2021 Nobel Prize in Physiology or Medicine was awarded to scientists David Julius and Ardem Patapoutian, who solved the mystery of the human sensation of touch and pain. They verified that cells sense pressure and elicit the sensation of touch through the electromechanical coupling effects of the proteins Piezo 1 and Piezo 2. We are inspired by this great discovery and have been thinking about making some new scientific advancements in the field of piezoelectric biomaterials and driving them towards real-world applications.
What are the challenges in developing piezoelectric biomaterials?
Currently, most piezoelectric materials are rigid and brittle, and some of them even contain toxic materials, making them unsuitable for implantation in the human body. Piezoelectric biomaterials are promising alternatives since they naturally exhibit biocompatibility, reliability, and resorbability. However, the piezoelectric strength of natural piezoelectric biomaterials such as bone and wood is very weak because of the disordered orientation. This makes them far from real-world applications. Hence, how to create order in piezoelectric biomaterials and improve their piezoelectric effect is extremely important. However, manipulating biomolecules at scale with the aligned orientation needed to function correctly has proved challenging.
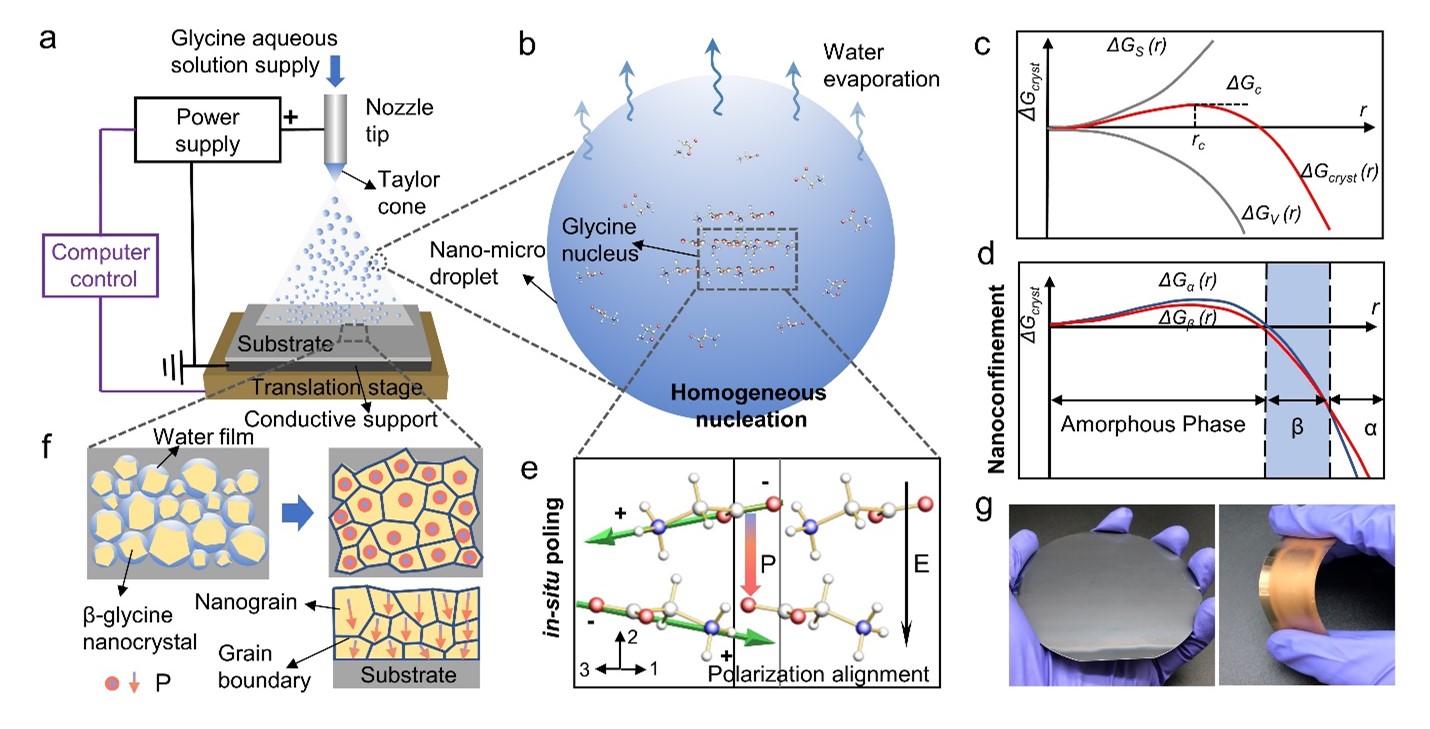
Here, we presented a generalizable strategy that enables biomolecules to self-assemble over a very large area with the same orientation via synergistic nanoconfinement, and in-situ electric filed. The biomolecular films show a compact and dense structure with uniformly high piezoelectric strength, which is superior to most reported bio-organic films. In addition, due to the nanoconfinement effect, the thermostability of these nanocrystalline films has been greatly improved compared with their bulk crystals (the failure temperature has been raised from 67 ℃ to 192 ℃).
Behind this research
In fact, in 2022, we had already published a study on biopiezoelectric tissues in Advanced Materials ("van der Waals Exfoliation Processed Biopiezoelectric Submucosa Ultrathin Films", DOI: https://doi.org/10.1002/adma.202200864). In this work, we systematically studied the biopiezoelectricity of Van der Waals layered small intestinal submucosa (SIS). For the first time, we quantitatively determined the inherent Piezoelectricity of SIS using the advanced piezoresponse force microscopy (PFM), and revealed the origin of its biopiezoelectricity. We proposed a van der Waals exfoliation process (vdWE) that utilizes weak van der Waals interactions in layered soft biological tissues to prepare ultra-thin films (100nm) with effective piezoelectric domains through simple mechanical peeling. However, the piezoelectricity of the soft tissue ultrathin film is still too small compared with the inorganic ceramics and organic polymers that are widely used at present, and because their piezoelectric direction is in-plane, its application scenarios are greatly limited.
Countless attempts and the final exciting discovery
Therefore, we have been thinking about whether high-performance piezoelectric biomaterials can be manufactured through fully controllable assembly at the molecular level. This is a great challenge. We tried various manufacturing methods and biomaterials, but did not achieve the desired results until a chance attempt at the end of 2021. During a discussion, we thought that it might be possible to try to prepare biomaterials using the recently built electrohydrodynamic spray deposition platform. Perhaps the in-situ electric field during the preparation process may have some surprising effects? After preparing the amino acid film, we immediately tested it using the most advanced PFM. We were surprised to observe that the prepared amino acid film exhibited a very high piezoelectric response at the nanoscale, while the deposited film was very dense and uniform.
However, at that point, we still did not know why the material exhibited such high piezoresponse, whether it had an aligned orientation at the macro scale, and what the underlying mechanism of its self-assembly was. In the subsequent material characterization, we were surprised that the thin film was not what they had imagined γ-Glycine crystalline phase, but a completely β Phase. However, β-Glycine has always been considered the most difficult to form among the three crystal forms of glycine and the most unstable environmental conditions. After being placed in humid air for several hours or heated to 67 ℃, it quickly transforms into non-piezoelectric α-Glycine. After countless days and nights of experimentation and exploration, we finally solved these difficult problems.

New technique and new mechanism for fabricating piezoelectric biomolecular films
We presented an active self-assembly strategy for the first time to prepare high-performance piezoelectric biomaterial films through synergistic nanoconfinement and in-situ poling. The nanoconfinement-induced homogeneous nucleation overcomes the interfacial dependency and allows the electric field applied in-situ to align crystal grains across the entire film. The β-glycine nanocrystalline films are fabricated based on a bio-organic film printer using the electrohydrodynamic spray method. We then proposed the potential nucleation and crystallization process as follows. The β-glycine nanocrystals are formed through homogeneous nucleation owing to the small size and substrate-free property of in-flight nano-micro droplets. As homogeneous nucleation is not influenced by solid-liquid interfaces, it is possible to manipulate the crystallization process by applying external electric fields, which also serve as the poling process. The in-situ electric field in the crystal growth process induces the domain alignment of β-glycine nanocrystals, suggesting the net polarization direction [020] is parallel to the electric field.
Our invented piezoelectric biomolecular films eventually can be applied in various high-performance biological electromechanical microdevices and transient bioelectronics, such as implantable biosensors, bioresorbable in vivo wireless charging power supply, smart chip,s and biomedical engineering.
Our next work will focus on three aspects: 1. Improving the flexibility of the film to match the modulus of biological tissue, 2. Low cost mass production of bioabsorbable piezoelectric thin films; 3. Promoting our products to real-world biomedical applications and benefit people.
More information: Zhuomin Zhang et al. Active self-assembly of piezoelectric biomolecular films via synergistic nanoconfinement and in-situ poling, Nature Communications (2023). DOI: https://doi.org/10.1038/s41467-023-39692-y
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in