In situ copper photocatalysts triggering halide atom transfer of unactivated alkyl halides for general C(sp3)-N Couplings
Published in Chemistry
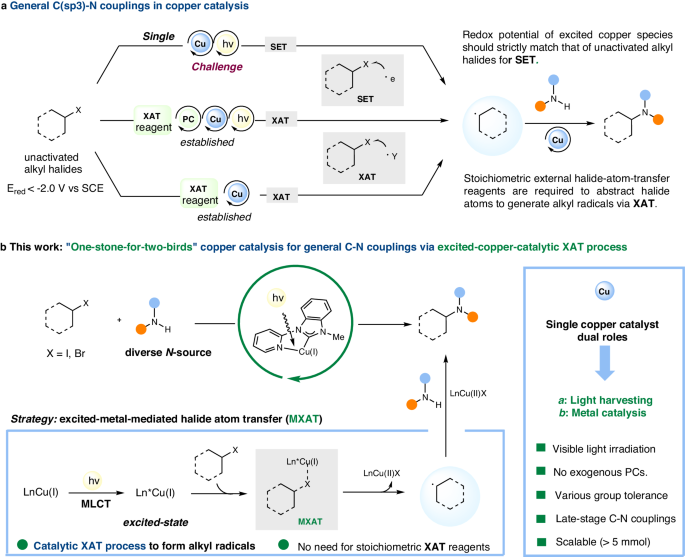
Building upon our previous work on utilizing a single bipyridine-ligated nickel photocatalyst for general C(sp2)-N couplings, we have expanded our investigation to encompass general C(sp3)-N couplings involving unactivated alkyl halides without the need for external photocatalysts. A significant challenge in this process lies in the low redox potential of unactivated alkyl halides, hindering the generation of alkyl radicals through an out-sphere single-electron process. Our aim is for the metal catalyst to independently reduce unactivated alkyl halides to general alkyl radicals without binding to N-nucleophiles, a crucial aspect for accommodating various nitrogen nucleophiles.
In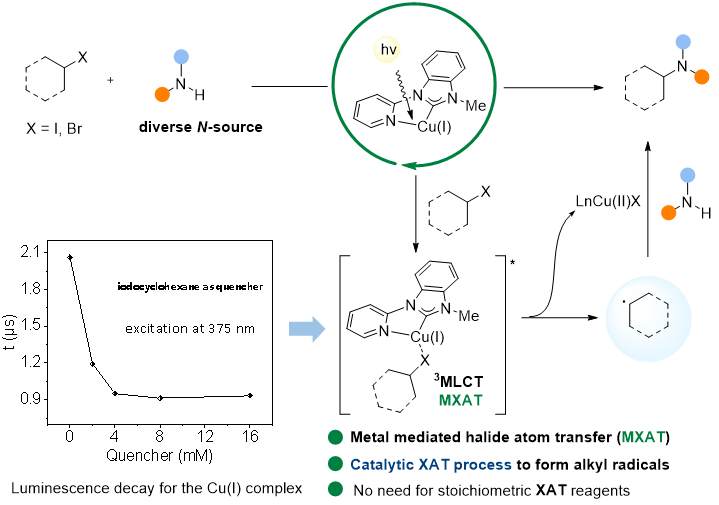 this study, we present a pyridyl-carbene copper catalyst generated in situ that facilitates general C(sp3)-N couplings using unactivated alkyl iodides and diverse nitrogen nucleophiles. Mechanistic investigations indicate that the copper catalyst does not require binding to N-nucleophiles for light-harvesting, allowing for the compatibility of various nitrogen nucleophiles in the coupling process. The electron transfer mechanism is supported by the formation of alkyl radicals and Cu(II) complexes, as evidenced by radical trapping, radical clock, and EPR experiments.
this study, we present a pyridyl-carbene copper catalyst generated in situ that facilitates general C(sp3)-N couplings using unactivated alkyl iodides and diverse nitrogen nucleophiles. Mechanistic investigations indicate that the copper catalyst does not require binding to N-nucleophiles for light-harvesting, allowing for the compatibility of various nitrogen nucleophiles in the coupling process. The electron transfer mechanism is supported by the formation of alkyl radicals and Cu(II) complexes, as evidenced by radical trapping, radical clock, and EPR experiments.
Transient absorption spectroscopy experiments revealed a single-electron transfer process occurring in the long-lived triplet excited states, as the singlet excited states have too short of a lifetime to react with alkyl iodides due to ultrafast intersystem crossing (260 ps). Surprisingly, quenching experiments with unactivated alkyl halides did not align with Stern-Volmer quenching behavior. The oxidation potential of the 3MLCT state of the copper catalyst was estimated to be -2.19 V vs SCE, insufficient for reducing unactivated alkyl iodides and bromides. We suspect that the single-electron transfer process occurs at the exciplex of the triplet excited states of the copper complex and the alkyl halide.
Our discovery of a new copper catalyst system for enabling general C(sp3)-N couplings with unactivated alkyl halides has the potential to advance new synthetic methodologies through the novel photo-excited copper-mediated halide atom transfer process.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
X:@LIN_Chemgroup