Increasing the dimensionality hierarchically….
Since the discovery of graphene as a molecularly thin, self-standing material with internal periodicity and its exceptional properties, organic chemists have been striving to build atomically thin two-dimensional polymers (2D-Ps) foreseeing their potential applications. In 1993, Roald Hoffmann mentioned as, “Organic chemists are masterful at exercising control in zero dimensions. One subculture of organic chemists has learned to exercise control in one dimension. These are polymer chemists, the chain builders. . . But in two or three dimensions, it’s a synthetic wasteland.” Despite such prejudices, chemists stepped up to the challenge of synthesizing 2D-Ps, but the path to achieving such perfect macromolecular sheets was fraught with many pitfalls. After several years of dedicated efforts, the synthesis of fully organic 2D-Ps from molecular precursors were realized by different means including on-surface, interfacial, and solution-phase polymerizations. Later, topochemical polymerization has been recognized as a convenient method for the synthesis of organic 2D-Ps in crystals and delamination of the lamellar 2D-P crystals in to atomically thin macromolecular sheets. Compared to other two-dimensional polymerization methods, topochemical approach for two-dimensional polymerization stands out as it allows for the unambiguous structure determination of the macromolecule, mechanistic understanding of the polymer growth and comprehension of structure-property relationships. However, despite these benefits, the growth in this domain in the realm of two-dimensional polymers has been rather slow, unlike the notable evolutions seen in 2D-COFs in the recent past. This is mostly due to the fact that topochemical 2D polymerization so far is entirely dominated by photochemical [4+4], [4+2], and [2+2] cycloadditions, which necessitates strict ready-to-react monomer packing, placing the reactive moieties in an ideal geometry for the successful transformation of the monomer to the 2D-P within the molecular plane. To realize novel topologies and diverse applications in topochemically accessed 2D-Ps, it is essential to expand the monomer scope and explore more reactions for topochemical 2D polymerization which is still in its infancy.
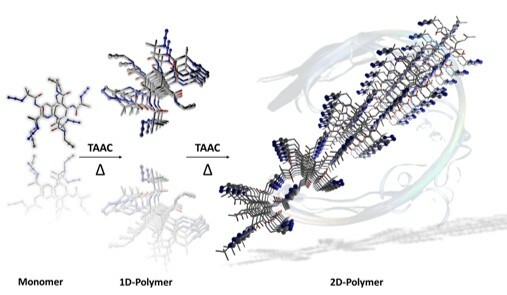
Our journey in topochemical reactions started with click polymerization of an azide-and-alkyne containing sugar derivative in the solid-state. Starting from there, with diligent efforts, we mastered topochemical azide-alkyne cycloaddition (TAAC) and extended this chemistry to make a library of one-dimensional polymers with variety of properties and applications. TAAC reactions are adaptable in terms of the geometric requirements as thermal motions of terminal reactive groups aid in achieving proximal geometry through rotation and reorientation. This would help in relaxing the stringent geometric criteria imposed by photochemical reactions. Well, we thought why can’t we broaden the scope of heat-induced TAAC reactions for achieving two-dimensional polymers?
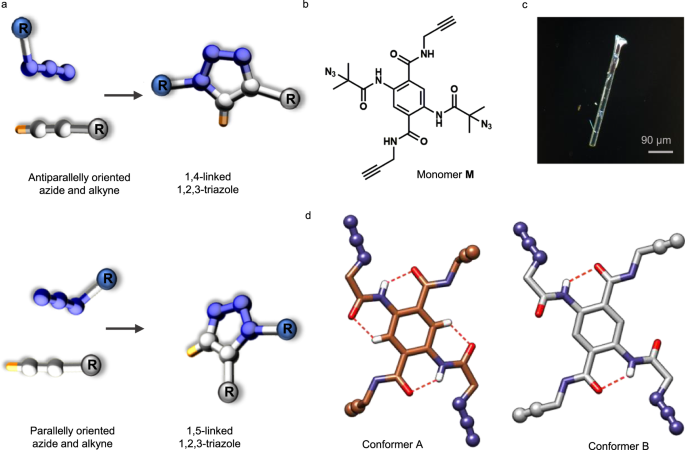
While pursuing this interest, the major challenge we dealt with was the right design of the monomer. We arrived finally at a rigid and compact single-benzene derived monomer containing two-pairs of azide and alkyne groups optimally placed for TAAC reactions in two mutually orthogonal directions. To our surprise, TAAC reactions proceeded in a step-wise manner, first transforming the monomer to a 1D-polymer and subsequently to a 2D-polymer. Thus, we could realize a hierarchical route towards the synthesis of a 2D-P for the first time via heat-induced TAAC polymerization. Additionally, the 2D-P comprise of two-different triazolyl linkages in two orthogonal directions as never been observed before. To know more about our approach for the 2D-P synthesis, from the design, to the polymerization and finally to the exfoliation of the 2D-P crystal to thin macromolecular sheets, check read our article in Nature Communications, https://doi.org/10.1038/s41467-024-51051-z
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Ask the Editor – Polymers
Got a question for the editor about Functional polymers? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in