Different Pre-organization for Different Traits: Three Different Polymorphs of a Polymer via TAAC Reaction
Published in Chemistry

Similar to allotropism shown by elements, chemical compounds exhibit polymorphism, a phenomenon wherein compounds crystallize in different forms and often show different properties. Polymers have revolutionized the world! Packing a polymer in different topologies could be a way to make different forms of the same polymer that have different properties. Unlike small molecules, crystallizing a polymer is not an easy task in view of the entropic factors and polydispersity.
Why not making crystal before polymerization? Sounds like ‘cart before the horse’. Isn’t it? But yes, it is indeed possible to obtain crystalline polymers via topochemical polymerization of monomers in crystal form. Though unsuccessful, there have been attempts to make different crystalline forms of the polymer via topochemical polymerization of different polymorphs of a monomer![i]
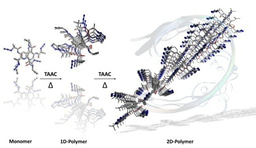
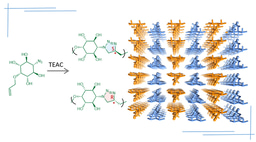
Our journey in this area started in 2012 when my first PhD student, Atchutarao, found that crystals of a monosaccharide having complimentary reacting groups viz. azide and alkyne undergo spontaneous topochemical azide-alkyne cycloaddition (TAAC) reaction in single-crystal-to-single-crystal (SCSC) fashion to give pseudopolysaccharide.[ii] The head-to-tail molecular arrangement and the proximity of the reacting groups azide and alkyne between adjacent monomers in the crystal are the essential criteria for the TAAC polymerization reaction.[iii] Later we have designed many reactive monomers and used TAAC methodology for the synthesis of many crystalline polymers.[iv] Right from the development of TAAC reaction, we were almost certain that we would be the first to make polymorphs of the polymer via topochemical reactions.
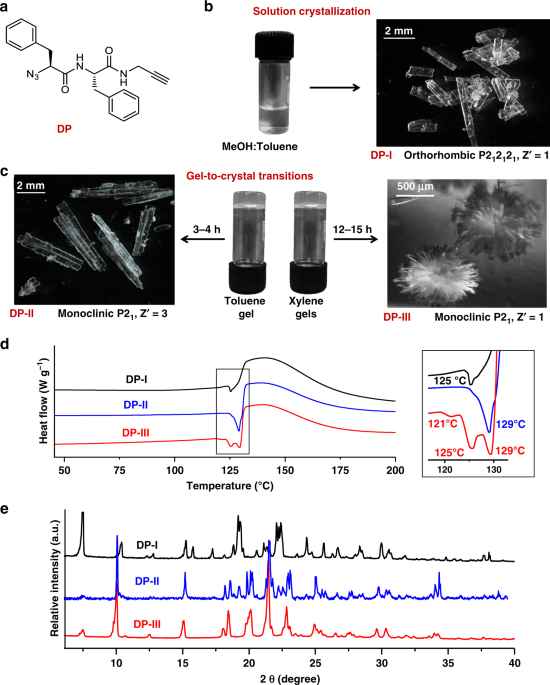
Mohanrao, my hard-working PhD student, was working on various phosphoinositols and despite his hardwork, he was not getting any exciting results. He was under tremendous frustration; I think all of us have gone through that stage. That is the time when he decided to work on TAAC reactions and he made the diphenylalanine-based monomer and he could do a SCSC polymerization of this monomer.[v] He is a great observer and he found that the monomer is also a gelator for various solvents. He also found that the gels slowly transform to crystals and that too to different polymorphic forms. Hema, another bright PhD student, was inducted to this project and she decided to analyze the structures of all the polymorphs and their topochemical reactivities. Hurray! all the three polymorphic forms tested were having very different packing and in all the forms, molecular arrangements were favorable for TAAC reaction. We all were excited and curious to know their actual reactivities. Finally, the hardwork paid off; we could make three different crystalline forms of the polymer.
Have a read of the full article and let us know about your opinion!
References
[i] Hsu, T.-J.; Fowler, F. W.; Lauher, J. W. Preparation and addition
of a tubular addition polymer: a true synthetic nanotube. J. Am. Chem. Soc. 134, 142–145 (2012)
[ii] Pathigoolla, A.; Gonnade, R. G.; Sureshan, K. M. Topochemical click reaction: spontaneous self-stitching of a monosaccharide to linear oligomers through lattice-controlled azide-alkyne cycloaddition. Angew. Chem. Int. Ed. 51, 4362–4366 (2012).
[iii] Pathigoolla, A.; Sureshan, K. M. A crystal-to-crystal synthesis of triazolyl-linked polysaccharide. Angew. Chem. Int. Ed. 52, 8671–8675 (2013)
[iv] Hema, K.; Sureshan, K. M. Topochemical azide-alkyne cycloaddition reaction. Acc. Chem. Res. 52, 3149–3163 (2019),
[v] Mohanrao, R.; Sureshan, K. M. Synthesis and reversible hydration of a pseudoprotein, a fully organic polymeric desiccant by multiple single-crystal-to-single-crystal transformations. Angew. Chem. Int. Ed. 57, 12435–12439 (2018).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
Interesting chemistry Great!
Thank you! It was really an exciting journey while developing this chemistry!
I had a wonderful experience while working on this project. Experienced Chemistry, Fun and Satisfaction all at the same time. Thank you Prof. Sureshan for your guidance and support.
After all it is yours and Mohans hardwork that paid off. Congratulations to you!