Is RNA editing one of the keys to deep subsurface survival?
Published in Microbiology

RNA is a key molecule in cellular biology whose essential function is to convert the genetic information encoded in cellular DNA into functional cell products, like proteins. Because of its importance, various RNA-based analyses, including metatranscriptomics, RNA stable isotope probing, and quantitative reverse transcription polymerase chain reaction (RT-qPCR) are commonly used to investigate the metabolic activities of microorganisms. These RNA-based approaches can capture complex gene suites expressed by organisms and can be used to estimate and compare expression levels in different samples.
Guaymas Basin is a young hydrothermally-influenced basin in the Gulf of California, Mexico, characterized by organic-rich sediments that along with conspicuous microbial mats, thermal mounds and chimneys form a complex hydrothermal landscape on the seafloor. Guaymas sediments reveal high taxonomic diversity, including novel taxa, as well as metabolic activities (e.g., chemolithoautotrophy) that were likely used by early microorganisms on Earth. Our study published in The ISME Journal (Mara et al., 2023; https://www.nature.com/articles/s41396-023-01492-z) benefited from deep subsurface sediment samples obtained during IODP 385 Expedition in Guaymas Basin. IODP385 Expedition drilled into seafloor sediments and volcanic subsurface basalts at eight sites with distinct temperature and geochemical profiles, and examined the subsurface biosphere by investigating microbial responses and adaptations to the different hydrothermal conditions that occur in the Basin. The sediment samples collected for metatranscriptomic analyses extended from 0.8 down to ~102 meters below seafloor (mbsf). Our analyses identified metabolic activities of Guaymas hydrothermal subsurface microorganisms that help them to sustain their metabolism in such a challenging environment.
Extracting RNA from those subsurface sediment samples was difficult considering the abundance of certain inhibitory compounds (e.g., oil hydrocarbons) that obscured RNA isolation. Nonetheless, we were able to successfully extract total RNA and create 19 cDNA libraries from sites U1545-U1552; our samples span distinct temperature profiles (cool/moderate/hot sites: 3oC-50oC) and biogeochemical regimes (e.g., 0.05 to 8.5 mM of H2S). While we find expression of genes for pathways predicted for hydrothermal ecosystems (methanogenesis, sulfate reduction, chemoautotrophy) we were also able to identify certain RNA modifications that appear to be central to survival for the Guaymas subsurface community (Figures 1 and 2).
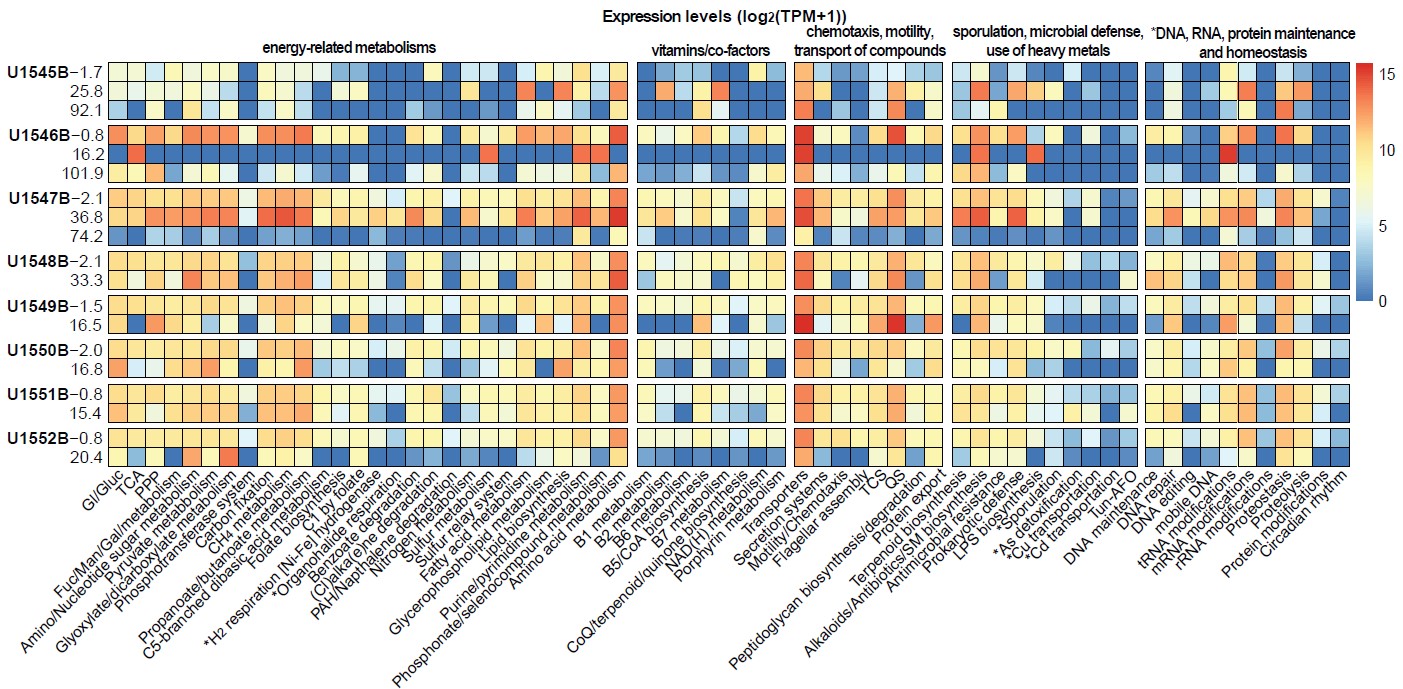
Figure 1. Prokaryotic metabolic and cellular processes identified at subsurface sediments from the eight sites (Y-axis) drilled in Guaymas Basin.
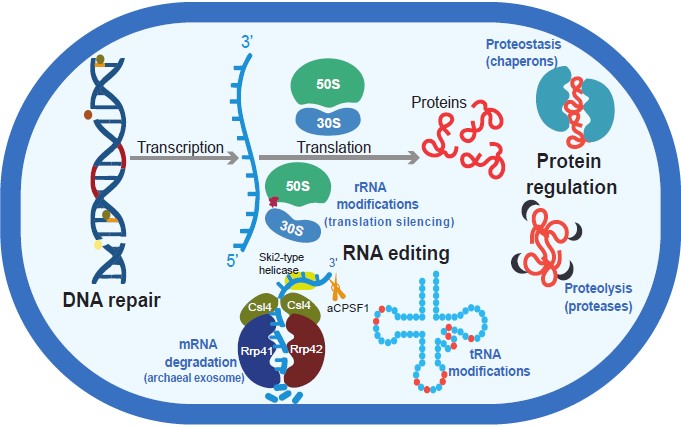
Figure 2. Overview of expressed genes for DNA maintenance and repair, RNA editing, and protein homeostasis and degradation used by Guaymas Basin subsurface Bacteria and Archaea.
RNA modifications, such as tRNA wobbling, mRNA degradation and ribosomal (rRNA) silencing are universal RNA editing processes in Bacteria, Archaea, and Eukaryotes, and have been demonstrated from studies of laboratory strains. However, in our study we were able to see their importance in an ecological context, within a natural microbial community.
Of particular interest is the fact that Guaymas subsurface hosts different archaeal taxa that belong to the Asgard superphylum, which was recently shown to be closely related to eukaryogenesis (Eme et al., 2023). We provide evidence for expression of genes affiliated with Asgard and other archaeal exosomes involved in RNA degradation whose function and structure share homology with eukaryotic exosomes. Overall, exosomes are sophisticated molecular machines that ensure only high-quality RNAs to be engaged in protein synthesis. This occurs through the degradation of “unwanted” RNA (RNA molecules no longer necessary for the cell), as well as RNA that has been processed incorrectly and contains defects (Vanacova and Stefl, 2007). In humans, mutations or misfunction of those central-to-life exosome machineries are related to various diseases (e.g., Pegtel and Gould, 2019). Because Asgard archaea share interesting homologies with eukaryotes, studies of archaeal RNA modifications from the deep biosphere may provide interesting insights into RNA editing, with potential use in other fields of biology. Likewise, the expression of Asgard genes associated with the nascent polypeptide-associated complex (NAC) that shares homology with the eukaryotic NAC, also merits further investigation. NAC binds to ribosomal RNA to protect the newly synthesized polypeptide chains from random degradation, and to ensure that these polypeptide chains are folding properly into proteins and are transferred into their designated cellular topology (e.g., integrated into the membranes, secreted outside the cell etc.). Mutations in NAC genes are lethal for many multicellular eukaryotes including mice, fruit flies (Drosophila melanogaster) and nematode worms (Caenorhabditis elegans), while intracellular levels of NAC subunits are reported to change dramatically in human diseases and genetic syndromes (e.g., Spreter et al., 2005 and refs therein; Deuerling et al., 2019; Zheng et al., 2022).
Other RNA modifications that we find in our Guaymas Basin metatrasncriptomes include expression of genes affiliated with tRNA modifications. The tRNA modifications, in addition to their role in tRNA wobbling, could provide protection against viral attacks. Indeed, tRNAs are targets of viruses and through modification of amino acids at certain tRNA positions prokaryotes could protect against those viral attacks. Viral activity in the deep biosphere and in hydrothermal vent systems is known.
Overall, subsurface Bacteria and Archaea employ diverse RNA modifications and protein homeostasis using a suite of chaperones (including also archaeal chaperonins called “thermosomes”), at sites and subsurface depths with contrasting biogeochemistry regimes and temperature profiles. This indicates that these RNA modifications, aside from their role in controlling essential housekeeping processes, can also be used for survival when cells are walking the fine line between growth, dormancy or even death due to carbon and energy limitations. Deep biosphere is a challenging setting where available energy sources decrease drastically with water and sediment depth (e.g., Biddle et al., 2006; Jørgensen 2012; Teske et al., 2021). Here, maintenance of cellular homeostasis under energy limitation requires constant fine tuning of cellular machinery, and dictates life decisions (e.g., active division and growth vs. sporulation and dormancy). Expressed RNA-editing enzymes are crucial to sustain “life in the slow lane.”
For details, please see our manuscript https://www.nature.com/articles/s41396-023-01492-z
References:
- Mara P, Zhou YL, Teske A, Morono Y, Beaudoin D, Edgcomb V. Microbial gene expression in Guaymas Basin subsurface sediments responds to hydrothermal stress and energy limitation. ISME J. 2023. doi: 10.1038/s41396-023-01492-z.
- Eme L, Tamarit D, Caceres EF, Stairs CW, De Anda V, Schön ME, et al. Inference and reconstruction of the heimdallarchaeial ancestry of eukaryotes. Nature 2023; 618(7967):992-999.
- Vanacova S, Stefl R. The exosome and RNA quality control in the nucleus. EMBO Rep. 2007; 8(7):651-7.
- Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019; 88:487-514.
- Spreter T, Pech M, Beatrix B. The crystal structure of archaeal nascent polypeptide-associated complex (NAC) reveals a unique fold and the presence of a ubiquitin-associated domain. J Biol Chem. 2005; 280(16):15849-54.
- Deuerling E, Gamerdinger M, Kreft SG. Chaperone Interactions at the ribosome. Cold Spring Harb Perspect Biol. 2019; 11(11):a033977.
- Biddle JF, Cardman Z, Mendlovitz H, Albert DB, Lloyd KG, Boetius A, et al. Anaerobic oxidation of methane at different temperature regimes in Guaymas Basin hydrothermal sediments. ISME J. 2012; 6:1018–31.
- Jørgensen BB. Shrinking majority of the deep biosphere. Proc Natl Acad Sci USA. 2012;109:15976–7.
- Teske A, Wegener, G., Chanton JP, White D, MacGregor B, Hoer D, et al. Microbial Communities under distinct thermal and geochemical regimes in axial and off-axis sediments of Guaymas Basin. Front Microbiol. 2021; 12:633649.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
It is exciting work and indeed insightful to document the expression of those quality checking and environment-responding control of gene expression. There is an issue of nomenclature, as it seems. RNA editing is a specific term used to describe base changes when or after a gene is transcribed to RNA. RNA editing events are inferred from base differences between genomic DNA and corresponding RNA sequences. Enzymes involved in this process include, for instance, adenosine deaminase acting on RNA (ADAR). RNA editing is more common in mitochondrial and chloroplast genes and rarely occurs in eukaryotes' nuclear genes.
RNA editing in dinoflagellates is quite extensive and diverse. References can be found in the following and subsequent publications:
Widespread and extensive editing of mitochondrial mRNAs in dinoflagellates.
J Mol Biol . 2002 Jul 19;320(4):727-39. doi: 10.1016/s0022-2836(02)00468-0.