Late-Stage Trifluoromethylthiolation of Benzylic C-H Bonds
Published in Chemistry
Trifluoromethylthio group (SCF3) has strongly electron-withdrawing power and high lipophilicity (πR = 1.44) and its insertion into organic molecules and pharmaceuticals could improve the cell membrane permeability and metabolic stability of the target molecules. As a consequence, the development of organic synthetic strategies accessing trifluoromethylthiolated compounds has gained considerable momentum in recent years (Acc. Chem. Res. 2015, 48, 1227; Chem. Rev. 2015, 115, 731; Acta Chimica. Sinica 2017, 75, 744). Although several trifluoromethyl-thiolated drugs (eg., tiflorex, toltrazuril and tiflorex in Fig. 1a) have been approved by FDA, the future development of such compounds depends on the evolution of synthetic strategies entailing versatility, diversity and availability.
In our previous work (Angew. Chem. Int. Ed., 2018, 57, 10357), we reported the first transition-metal-free, site-specific umpolung trifluoromethylthiolation of tertiary alkyl ethers. Under the optimal conditions, a large number of tertiary alkyl trifluoromethyl sulfides were obtained.
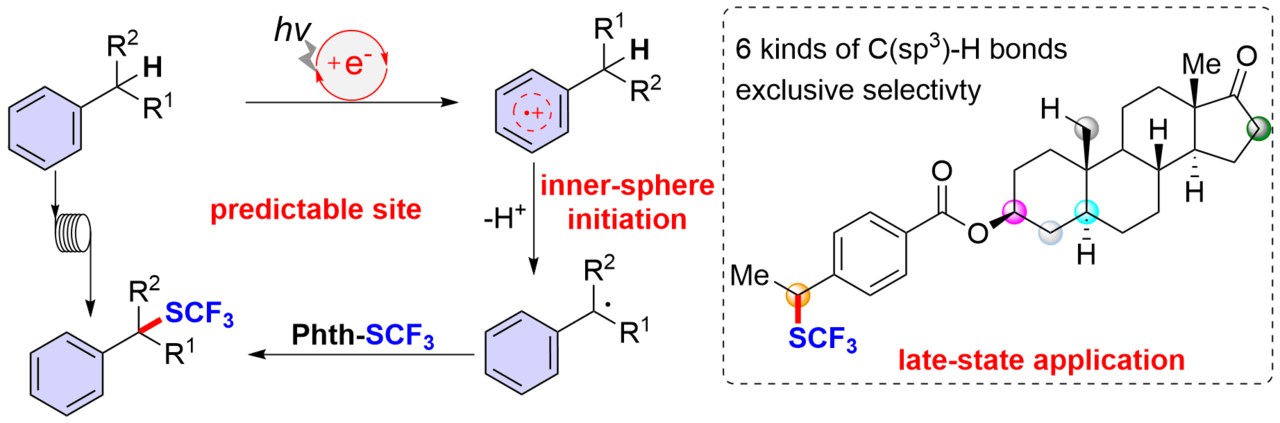
As an ongoing interest in this topic, our recent work, published in Nature Communications, disclosed an organophotoredox catalyzed reaction for site-selective benzylic C–H bond trifluoromethylthiolation of a wide variety of alkyl arenes and heteroarenes. The only successful benzylic C–H trifluoromethylthiolation strategy (Org. Lett. 2014, 16, 3372) is copper-catalyzed oxidative trifluoromethylthiolation with AgSCF3 but the reaction needs a large excess of simple toluene analogs (60 equivalents compared to AgSCF3). In this event, there are two significant challenges: a) site-selective benzylic C-H trifluoromethylthiolation; b) late-stage trifluoromethylthiolation. Hence, we report the development of a metal free, photoredox inner-sphere HAT process which predictably generates, from natural products or drug derivatives, a benzylic radical which can be trifluoromethylthiolated, avoiding the use of oxidants and HAT reagents (Fig. 1). This protocol affords structurally diverse benzylic trifluoromethyl sulfides with moderate to good yields. The broad scope, excellent functional group compatibility, and predictable regioselectivity allow for efficient late-stage benzylic C–H trifluoromethylthiolation of a variety of drug candidates and complex molecules. Large scale trifluoromethylthiolation can be achieved with continuous flow photoredox technology, which further demonstrate the practicability of the present method. We believe that this strategy will expedite precise benzylic C–H functionalization in complex molecules and that it will promote the construction of a library of benzylic trifluoromethyl sulfide leads for drug discovery.
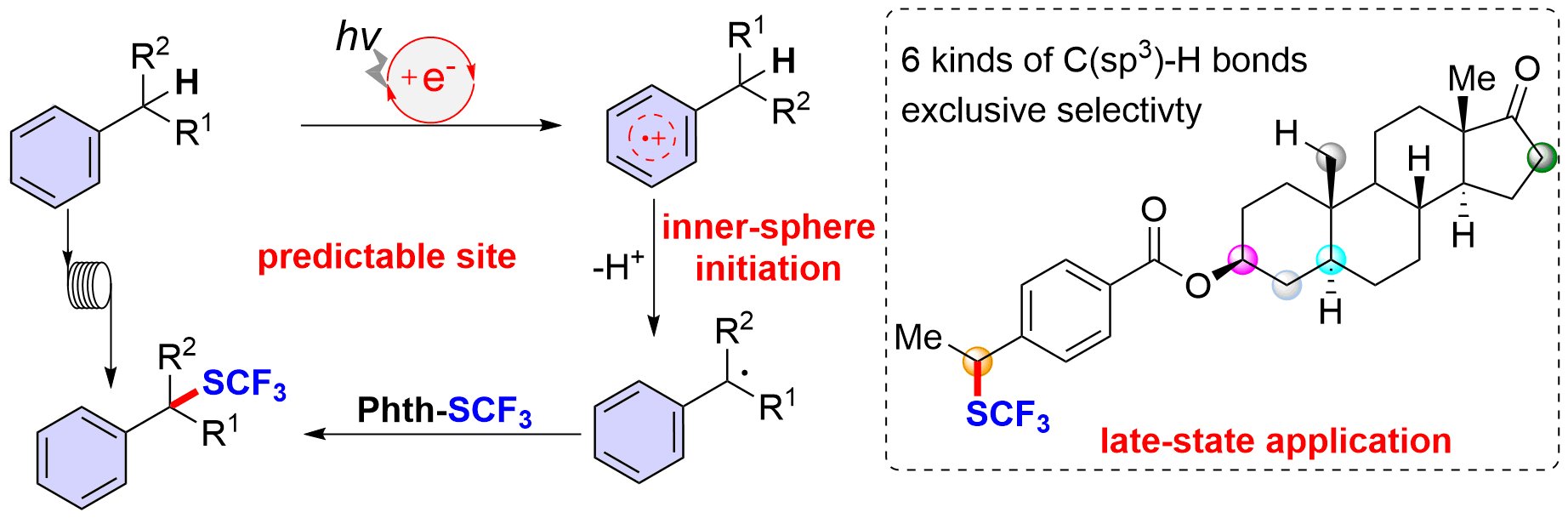
Fig. 1
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in