Nanomedicine Toward Overcoming Virus Diseases
Published in Bioengineering & Biotechnology

Infectious disease caused by viruses as represented by current coronavirus disease 2019 (COVID-19) has been a threat to human, and how to overcome the viral diseases is a major challenge in science. Nanocarriers that recognize reporter molecules at virus-infected sites to release drugs, are promising to achieve nanomedicine for curing the viral diseases. We focused on guanosine triphosphate (GTP) as a reporter molecule for designing nanocarriers targeting virus-infected cells. GTP is an intracellular molecule involved in many essential biological processes, such as cell division, nucleotide synthesis, and cell signaling. In the cell division process, thetubulin heterodimer (THD), which constitutes microtubule (MT), uses GTP as an energy source to induce its polymerization and depolymerization1. GTP is also used as a component for the self-replication of RNA viruses such as coronaviruses2,3. Notably, GTP is abundant in certain diseased cells (1.5–4.5 mM) such as rapidly proliferating cancer cells and RNA virus-infected cells, whereas the concentration of GTP, unlike that of ATP, is negligibly low in normal cells (< 0.3 mM)4. Therefore, GTP-responsive nanocarriers have the great potential to efficiently cure cancer and RNA virus-induced diseases including COVID-19. However, it has been difficult to design nanocarriers that solely respond to GTP because there are molecules in the cell, such as ATP5, that have similar molecular structure to GTP.
In this paper, we successfully constructed GTP-responsive nanocapsules (NCGTP/GTP*) composed of THD. THD is composed of α-tubulin and β-tubulin. GTP attached to the β-tubulin unit is known to be hydrolysable to GDP, which causes a structural change of THD. Nonhydrolysable GTP analogue (GTP*) can also bind to the β-tubulin unit of THD to afford THDGTP*. Both THDGTP and THDGTP* have been reported to self-assemble into MTGTP and MTGTP*, respectively, at 37 °C. The construction of NCGTP/GTP* was realized based on two serendipitous findings. We tried to modulate the stability of MT against depolymerization by changing the THDGTP/THDGTP* molar ratio. When we heated a mixture of THDGTP and THDGTP* (THDGTP/THDGTP* = 17/83) at 37 °C, unexpectedly, a leaf-like 2D nanosheet (NSGTP/GTP*) was obtained rather than MT. Since such 2D nanosheets have recently attracted particular attention, we then attempted to functionalize NSGTP/GTP* by using photoreactive molecular glue (Glue),6 which has been developed for other purposes in our laboratory to functionalize biomolecules such as proteins, nucleic acids, and phospholipid membranes. Glue has multiple guanidinium ions and photoreactive benzophenone groups, which is designed to covalently functionalize proteins under physiological conditions. To our surprise, by treating with Glue, NSGTP/GTP* was transformed into spherical NSGTP/GTP*. According to the MD simulations,7 we found that the adhesion of Glue to THD decreases the surface charge of THD and flatten the NSGTP/GTP* structure. This result suggests that the adhesion of Glue causes the stacking of NSs.
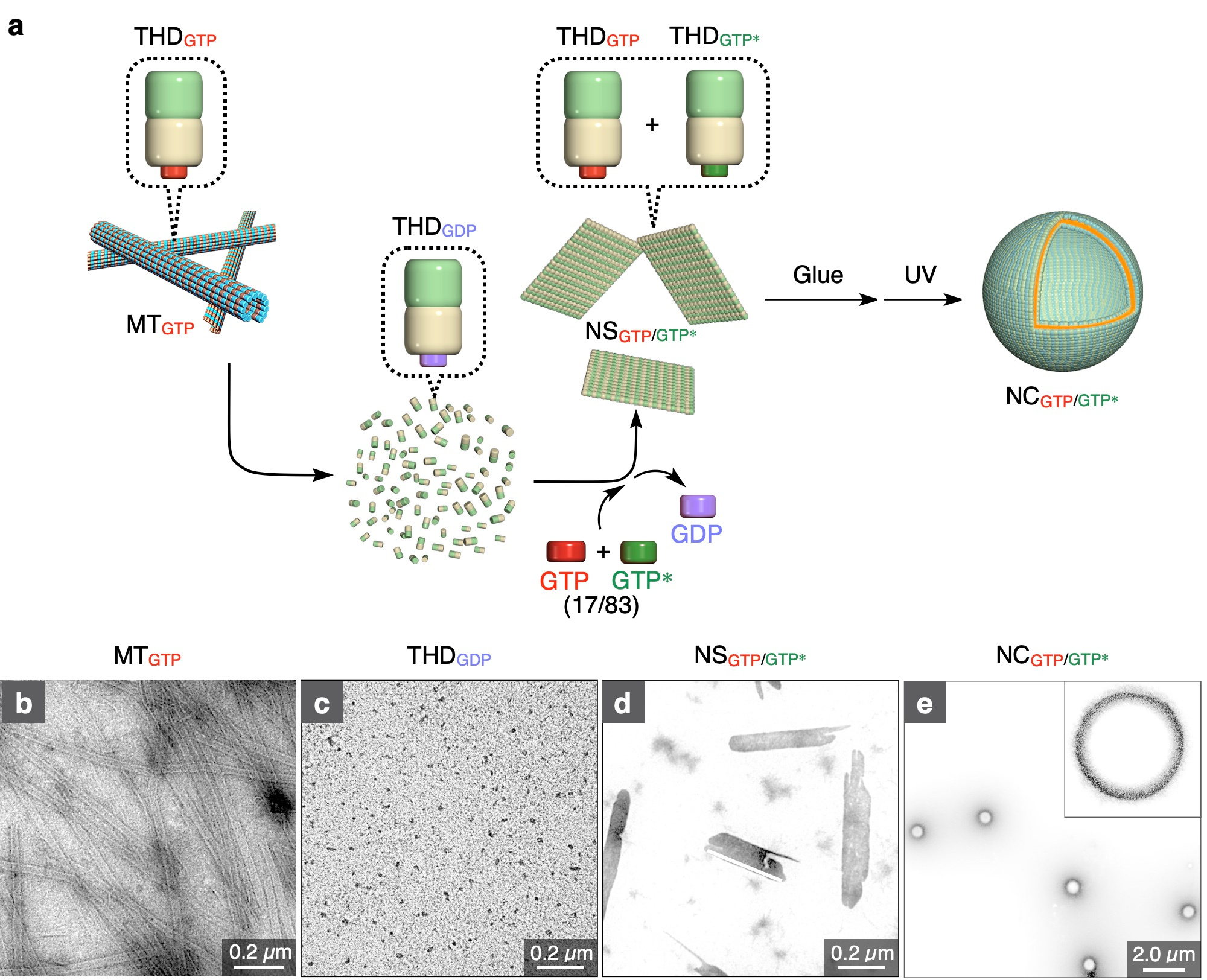
Figure 1. a, Schematic illustration of the multistep procedure for the synthesis of NCGTP/GTP* from MTGTP. MTGTP is depolymerized into THDGDP, which is incubated with a mixture of GTP* (83 mol%) and GTP (17 mol%) to form nanosheet NSGTP/GTP*. Upon treatment with photoreactive molecular glue (Glue), NSGTP/GTP* is transformed into spherical NCGTP/GTP*. b−e, TEM images of MTGTP (b), THDGDP (c), NSGTP/GTP* (d), and NCGTP/GTP* (e).
Because the nanocapsule NCGTP/GTP* is composed of GTP-responsive THD units, we considered that NCGTP/GTP*should also show GTP-responsive behaviors. Indeed, when we added 0.5 mM GTP to NCGTP/GTP*, the spherical structure collapsed. The collapse is induced probably because GTP* bound to THD in NCGTP/GTP* is replaced by GTP, which is subsequently hydrolyzed to GDP, thereby inducing the conformational change of THD. Notably, NCGTP/GTP*was not disrupted in the presence of low concentrations of GTP (<0.2 mM), suggesting that NCGTP/GTP* is a useful nanocarrier targeting cells overexpressing GTP, such as virus-infected cells. Since frequently proliferating cancer cells overexpress GTP, we tried to evaluate the performance of GTP-responsive NCGTP/GTP* as a drug delivery carrier by encapsulating doxorubicin, an anticancer drug. When doxorubicin-loaded NCGTP/GTP* was incubated with cancer cells for 2.5 hours, as observed by confocal laser scanning microscopy, doxorubicin was taken up into the cells efficiently. After the 24-hour incubation, the cell death resulted.
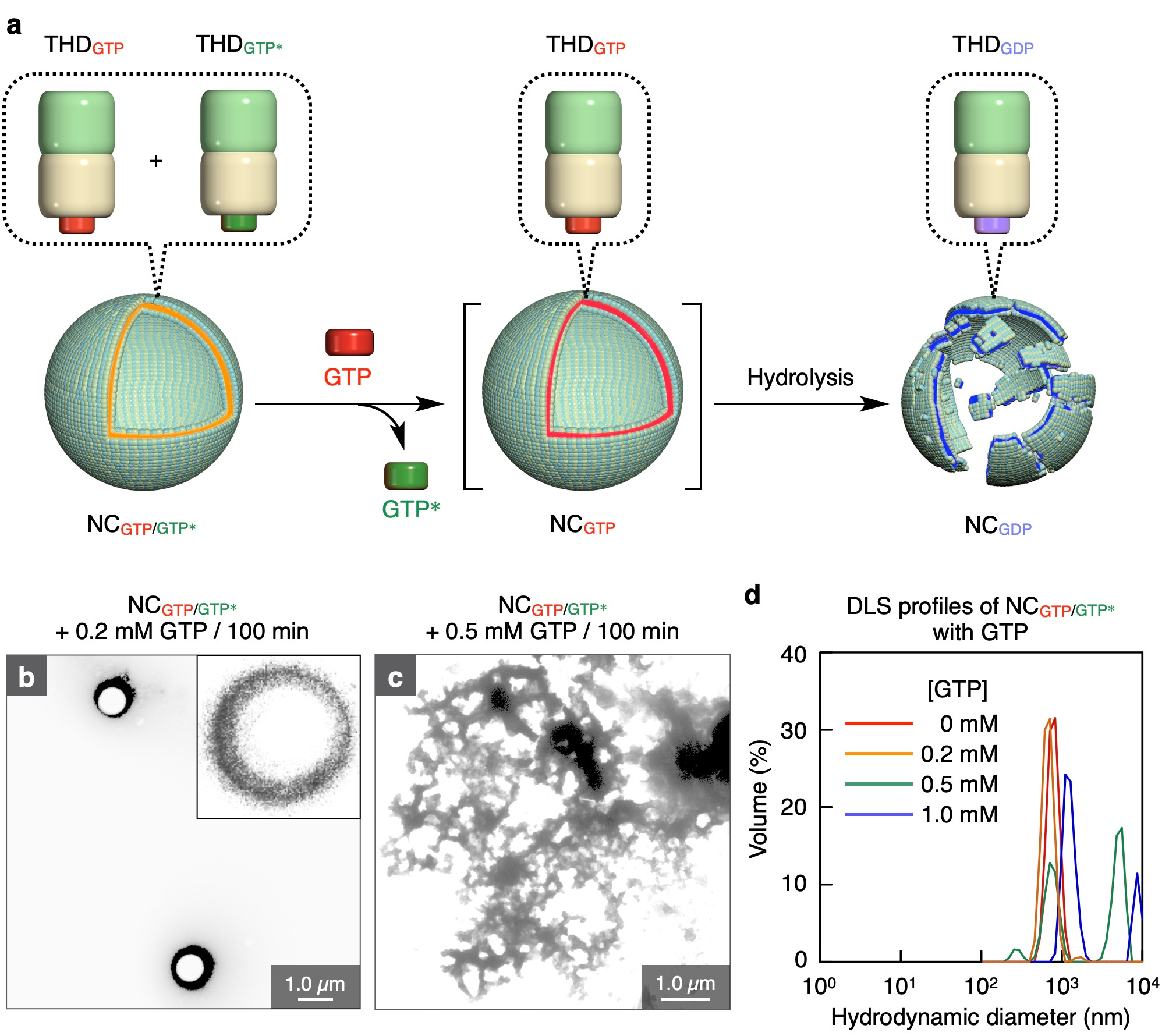
Figure 2. a, Schematic illustration of the GTP-responsive collapse of NCGTP/GTP*. b,c, TEM images of NCGTP/GTP* upon mixing with GTP at its concentrations of 0.2 mM (b) and 0.5 mM (c). d,Size distribution NCGTP/GTP* upon a titration with GTP (0−1.0 mM) analyzed by DLS.
In summary, we demonstrated that the drug delivery system using GTP-responsive NCGTP/GTP* composed of THD is useful for targeting cells overexpressing GTP. In the future, we plan to design NCGTP/GTP* encapsulating antiviral drugs for targeting cells infected by coronaviruses and influenza viruses for curing the viral diseases.
- Mitchison, T. & Kirschner, M. Dynamic instability of microtubule growth. Nature 312, 237–242 (1984).
- van Dijk, A. A. & Makeyev, E. V. Initiation of viral RNA-dependent RNA polymerization. Gen. Virol. 85, 1077–1093 (2004).
- Hu, B., Guo, H., Zhou, P. & Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Rev. Microbiol. 19, 141–154 (2020).
- Inaoka, T. & Ochi, K. RelA protein is involved in induction of genetic competence in certain Bacillus subtilis strains by moderating the level of intracellular GTP. Bacteriol. 184, 3923–3930 (2002).
- Smethurst, P. A. & Griffin, M. Measurement of tissue transglutaminase activity in a permeabilized cell system: its regulation by Ca2+ and nucleotides. J. 313, 803–808 (1996).
- Uchida, N. et al. Photoclickable dendritic molecular glue: noncovalent-to-covalent photochemical transformation of protein hybrids. Am. Chem. Soc. 135, 4684–4687 (2013).
- Garzoni, M., Okuro, K., Ishii, N., Aida, T. & Pavan, G. M. Structure and shape effects of molecular glue on supramolecular tubulin assemblies. ACS Nano 8, 904–914 (2014).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in