NPB Editor's Choice
Published in Healthcare & Nursing, Cell & Molecular Biology, and Biomedical Research
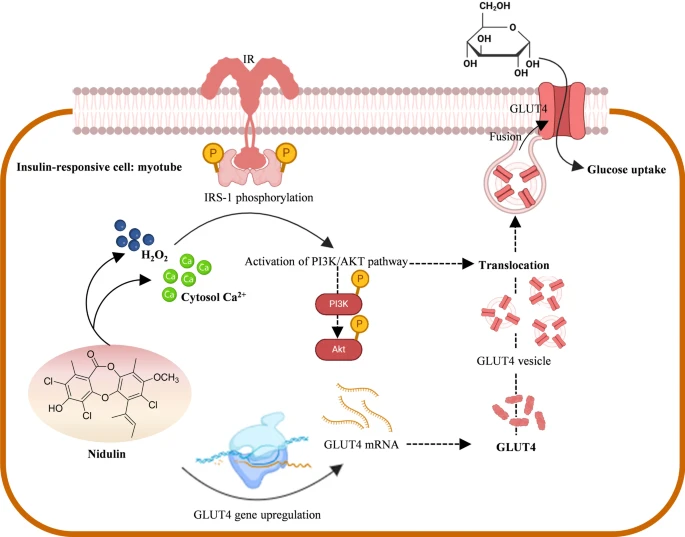
Nidulin—a fungal depsidone capable of substantially activating the PI3K/AKT signaling pathway independent of the insulin receptor—effectively transforms L6 myotubes into highly efficient glucose-clearing systems, even under conditions of palmitate-induced insulin resistance. By integrating redox and Ca²⁺ signaling to promote both GLUT4 translocation and transcriptional upregulation of GLUT1 and GLUT4, nidulin exhibits robust insulin-mimetic activity without requiring a functional insulin receptor. In vivo studies have already indicated initial tolerability, positioning it as a strong candidate for advancement into animal models of type 2 diabetes. Given its distinct mechanism of action, nidulin represents a promising first-in-class small-molecule insulin sensitizer with potential for monotherapy or combination regimens. For researchers seeking novel therapeutic candidates, nidulin warrants immediate consideration for inclusion in preclinical development pipelines.
Follow the Topic
-
Natural Products and Bioprospecting

This is a single blind peer-reviewed open access journal that devoted to rapidly disseminate research results in all areas of natural products.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
For researchers seeking novel therapeutic candidates, nidulin warrants immediate consideration for inclusion in preclinical development pipelines.