Precise recognition of benzonitrile derivatives with supramolecular macrocycle of phosphorylated cavitand by co-crystallization method
Published in Chemistry
In the field of structure-based drug design, molecular docking plays a central role in facilitating the establishment of specific lock-key interactions between a target protein and a drug molecule. The importance of molecular docking in the drug discovery process stems from its ability to facilitate precise recognition between a fragment of a potential drug compound and its target receptor, a process that relies predominantly on computational strategies. However, the prospect of exploiting the rigid cavity structure of supramolecular macrocycles to mimic the process of molecular docking through co-crystallization experiments, as opposed to traditional computational method, promises to increase the accuracy of guest recognition with specific fragments, thus opening up an exciting perspective in the field.
Recently, the group of Lin Chen and Jiang Juli at the School of Chemistry and Chemical Engineering, Nanjing University, Jiangsu Province, China, reported a phenylphosphine oxide-bridged aromatic supramolecular macrocyclic F[3]A1-[P(O)Ph]3, which achieves precise recognition of benzonitrile and its derivatives by mimicking the molecular docking technique using a co-crystallization method (Figure 1).
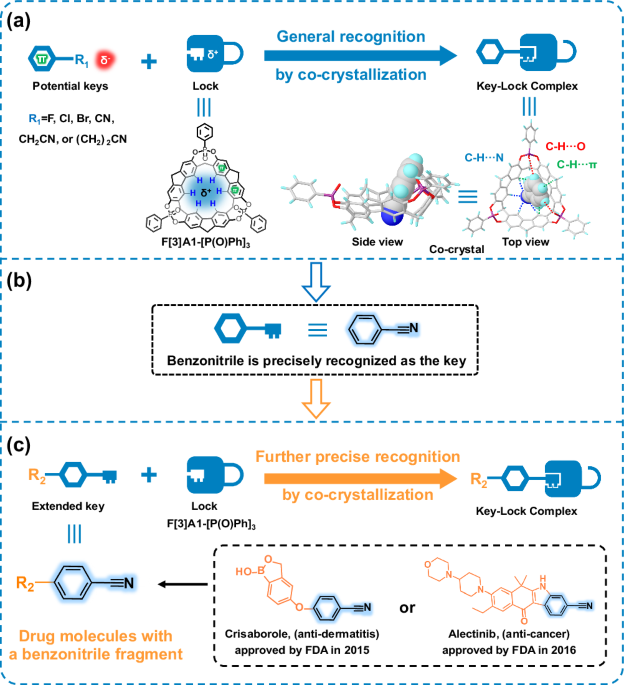
![Precise recognition studies of F[3]A1-[P(O)Ph]3 with guest molecules by co-crystallization to form key-lock complexes. Figure 1: Precise recognition studies of F[3]A1-[P(O)Ph]3 with guest molecules by co-crystallization to form key-lock complexes. (a) The potential guest molecules were generally recognized. (b) Benzonitrile was precisely recognized as the key. (c) More complicated guest molecules with a benzonitrile fragment, including drug molecules crisaborole (anti-dermatitis) and alectinib (anti-cancer), were further precise recognition.](https://images.zapnito.com/cdn-cgi/image/metadata=copyright,fit=scale-down,format=auto,quality=95/https://images.zapnito.com/uploads/eeDqUekCTJejhSTJEJT4_figure%201.png)
Figure 1: Precise recognition studies of F[3]A1-[P(O)Ph]3 with guest molecules by co-crystallization to form key-lock complexes. (a) The potential guest molecules were generally recognized. (b) Benzonitrile was precisely recognized as the key. (c) More complicated guest molecules with a benzonitrile fragment, including drug molecules crisaborole (anti-dermatitis) and alectinib (anti-cancer), were further precise recognition.
Based on the previously reported macrocyclic molecule 2,7-OH-F[3]A1 from Prof. Chuan-Feng Chen’ group, a bridged phenylphosphine oxide group was introduced at the middle edge of 2,7-OH-F[3]A1, resulting in the formation of a macrocycle with a fully completely conformation, named F[3]A1-[P(O)Ph]3. The supramolecular macrocyclic F[3]A1-[P(O)Ph]3 has a bowl-shaped geometry composed of three fluorene units, which is a completely locked conformation that leads to easy crystallization. Density functional theory (DFT) calculations on the crystal structure of F[3]A1-[P(O)Ph]3 have been performed to map the electrostatic potential (ESP) of F[3]A1-[P(O)Ph]3, showing that the hydrogen atoms at the lower edges of F[3]A1-[P(O)Ph]3 form a triangular positively charged region. The guest molecules containing benzene sheets with negative charges at the end of the molecules, such as chlorobenzene, bromobenzene, benzonitrile, p-fluorotoluene, p-hydroxy-benzyl cyanide, and 3-phenylpropionitrile, were selected as the key structure and co-crystallized with F[3]A1-[P(O)Ph]3 as the lock structure in an attempt to obtain the co-crystals with the key-lock combination mode. The results show that only benzonitrile molecules form a key and lock structure with F[3]A1-[P(O)Ph]3. The chlorobenzene or bromobenzene molecules exist only below the F[3]A1-[P(O)Ph]3 cavity. Although the p-fluorotoluene molecule is present in the macrocyclic cavity, the C-F bond length is relatively short and there is no interaction with the hydrogen atoms at the lower edge of F[3]A1-[P(O)Ph]3. The p-hydroxy-benzyl cyanide, and 3-phenylpropionitrile also did not form a key-lock combination mode with F[3]A1-[P(O)Ph]3, and were oriented towards the top of the cavity due to the long size of the terminal group. The observed host-guest interactions between F[3]A1-[P(O)Ph]3 and benzonitrile prompted us to further explore more complex benzonitrile derivatives. As expected, 12 structurally diverse guest molecules with benzonitrile fragments each formed key-lock complexes with F[3]A1-[P(O)Ph]3. This result demonstrates the universal adaptability of macrocyclic F[3]A1-[P(O)Ph]3 as a lock.
The presence of benzonitrile fragments in drugs is a common occurrence, so F[3]A1-[P(O)Ph]3 could possibly form enhanced key-lock binding modes with complex benzonitrile-based drug compounds, providing an experiential base for structure determination of drugs containing benzonitrile fragments. Thus, structurally complex drug molecules containing benzonitrile fragments, such as crisaborole (anti-dermatitis drug) and alectinib (anti-cancer drug), have also been accurately identified by co-crystallization methods for specific binding to F[3]A1-[P(O)Ph]3. We report here the high-resolution crystal structure of the drug molecule alectinib. It should be emphasized that the occupancy of the guest molecule was 100% in all the crystal data obtained, and the co-crystallographic single crystal data were also satisfactory.
The precise identification of the supramolecular host macrocycle with the guest molecule, in particular with the drug molecule, has been successfully achieved using the co-crystallization method, which establishes a far-reaching and effective experimental method and provides an empirical basis for the structural determination of benzonitrile fragment-containing drugs.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in