Retinoic acid blocks a chain of cancer-promoting signals in Acute Myeloid Leukemia
Published in Cancer, Research Data, and Cell & Molecular Biology
Nucleophosmin-1 (NPM1): The Cell Multitasking Assistant
Under normal conditions, NPM1 acts as a helpful assistant in the cell. It works inside a compartment called the nucleolus; a small control center tucked within the nucleus. Think of NPM1 as a project manager, handling several important tasks at once: helping cells grow, building ribosomes (the factories that make proteins), and keeping watch over other important players like ARF and P53, which help control cancer. But like in any good mystery, things don’t always go as planned.
When One Mutation Flips the Whole Script
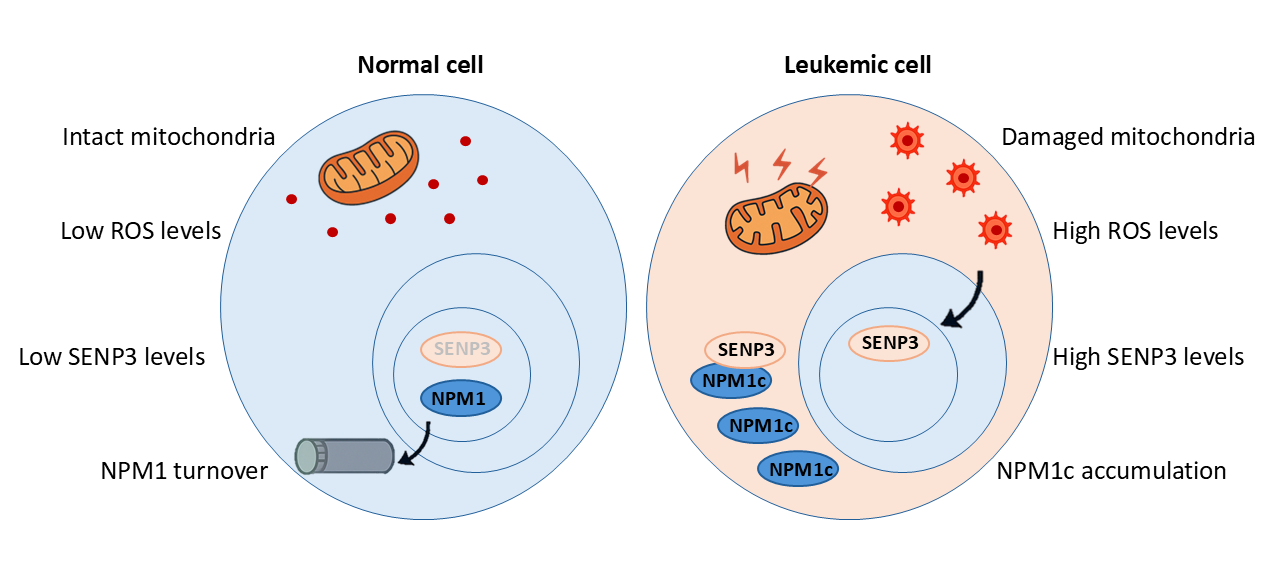
In about one-third of Acute myeloid leukemia (AML) cases, something unsual happens to NPM1: it mutates. This isn’t just a minor glitch; this mutation completely changes the whole protein behavior. Instead of staying in the nucleolus, the mutated version, known as NPM1c, escapes into the cytoplasm, the jelly-like fluid that fills the cell. This escape is not just a change of scenery; it is a big problem. As such, NPM1c can no longer support the cell usual safety systems. It shuts down key protectors like P53 and ARF, which normally act as brakes to stop on uncontrolled growth. Even worse, NPM1c interferes with the cells emergency response centers, called PML nuclear bodies. These structures play a crucial role in keeping the cells powerhouses, mitochondria, healthy. Without them, the cells ability to handle stress and stay balanced falls apart.
When the Cells Powerhouses Start to Fail
Mitochondria are of the powerhouses of our bodies. These tiny structures produce the energy our cells need to function. But mitochondria are also sensitive and easily thrown off balance. When NPM1c disrupts their function, they begin to malfunction. They begin to overheat and release harmful substances called reactive oxygen species (ROS). And here’s where things take an even darker turn. ROS doesn’t just signal damage. It activates a protein called SENP3, which acts like a cleaner, removing special molecular tags called SUMO from other proteins. SENP3 targets NPM1c and removes its SUMO tags, which ironically helps NPM1c survive longer instead of breaking down, and only adds to the chaos (See Figure 1).
So now we have a toxic feedback loop:
- NPM1c damages mitochondria.
- Damaged mitochondria produce ROS.
- ROS activates SENP3.
- SENP3 shields NPM1c from destruction.
- NPM1c accumulates, and the loop repeats.
This self-reinforcing cycle lets leukemia cells survive and grow.
Plot Twist! Retinoic Acid Enters the Scene
Retinoic acid (RA) is a natural compound made from vitamin A. You might recognize it from skin care products, but in medicine, RA has a much bigger role, as it cures a subtype of blood cancer called APL. It works by breaking apart a harmful fusion protein called PML-RARA that causes the disease. That success made us ask: could RA could RA help against NPM1c AML too?
In clinical trials, the results were promising. When RA was given alongside chemotherapy or drugs that affect DNA, patients with NPM1c lived longer survival and had better chances of going into remission.
Breaking the Vicious Cycle: Retinoic Acid’s Role
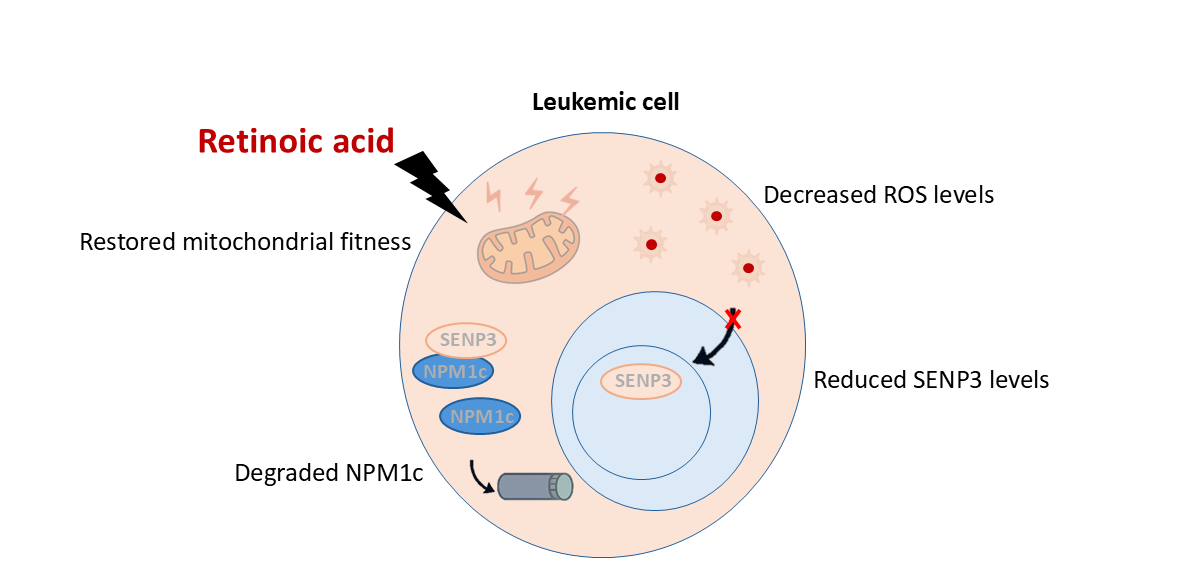
Our research uncovered how RA works: it acts like a reset button for the mitochondria. Once inside the cells, RA rapidly restores normal mitochondrial function, halting the release of reactive oxygen species and clearing the oxidative signaling distress. As ROS levels drop, SENP3 becomes unstable and is broken down by the cell recycling system (called the proteasome). In the absence of SENP3, NPM1c gets tagged with the molecular “destroy me” signal called SUMO. This tag is recognized by another helper protein, RNF4, which adds another tag called ubiquitin. Together, these signals send NPM1c to the proteasome, where it’s broken down. With NPM1c gone, the harmful cycle is stopped. Important cancer-fighting proteins like ARF and P53 can now return to normal levels, helping the cell either stop growing uncontrollably or die off, restoring balance and offering hope for treating this type of leukemia (See Figure 2).
From Petri Dishes to Mice: RA Is Making a Difference
In our study, we also tested RA in mice given human leukemia cells, either from lab-grown AML cells or directly from patients. These mice were treated with RA alone or in combination with the chemotherapy drug AraC. The results were clear and consistent: the mitochondrial health was restored, the harmful chemical signals ROS levels went down, and both SENP3 and the faulty NPM1c were eliminated. As a result, the amount of leukemia in the mice dropped significantly.
So why does It Matter?
Cancer is often treated by hitting it hard with toxic drugs, hoping the cancer cells die off before too much damage is done to healthy ones. But our findings point to a smarter, more targeted approach: fix what is out of balance inside the cell. In NPM1-mutated AML, that imbalance is a vicious loop between a mutated protein, damaged mitochondria, and chemical stress.
RA, a natural molecule our bodies naturally make from vitamin A, can break that cycle and help restore balance in the cell.
It’s not a magic bullet. Not every patient with AML will respond to RA, especially those with additional mutations like FLT3-ITD. But for patients with NPM1 mutations, especially older adults who can’t tolerate aggressive chemotherapy, this strategy could offer a gentler and more effective treatment option.
Looking at The Bigger Picture
This story is not about one protein or one drug. It’s a story about the power of understanding how our cells work. When we truly understand what goes wrong in a disease, down to the tiniest molecules, we can find smarter, safer ways to treat it. Instead of using harsh treatments that attack everything, we can design precise solutions that fix the real problem. It is a shift from simply trying to kill cancer to actually correcting the systems that have taken over. That is the future of medicine, it starts with understanding.
Follow the Topic
-
Leukemia

This journal publishes high quality, peer reviewed research that covers all aspects of the research and treatment of leukemia and allied diseases. Topics of interest include oncogenes, growth factors, stem cells, leukemia genomics, cell cycle, signal transduction and molecular targets for therapy.
Your space to connect: The Myeloid cell function and dysfunction Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine and Cell Biology!
Continue reading announcement





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in