Revolutionizing Microreactors and Beyond: Capillary Force-Driven Droplet Encapsulation
Published in Physics

Droplets, in their myriad forms and functions, have become indispensable players in the world of microreactors, drug delivery systems, crystallization studies, and cell culture platforms. These tiny, self-contained units offer tremendous potential but also pose significant challenges, particularly concerning droplet encapsulation and evaporation resistance. Traditional methods have struggled to provide a one-size-fits-all solution for encapsulating droplets of varying sizes, often leading to contamination, substrate dependency, and rapid evaporation. Contamination can compromise the reliability of chemical reactions, while substrate dependence limits the flexibility of droplet-based assays. Rapid evaporation, on the other hand, affects crystal growth, cell viability, and droplet sensor stability. Solving these issues requires a versatile and scalable encapsulation technique that can be applied across a broad spectrum of droplet sizes and volumes. However, capillary force-assisted cloaking, powered by hydrophobic nano-micron-sized particles and liquid-infused surfaces, has emerged to tackle these issues head-on. In our article, we delve into a simple yet elegant approach of capillary rise and explore its transformative potential across diverse scientific domains.
The Promise of Capillary Force-Assisted Cloaking:
Capillary force-assisted cloaking holds the potential to revolutionize the way we encapsulate droplets. This technique combines the hydrophobic nano-micron-sized particles and oil-infused surfaces to create uniform solid and liquid shell encapsulations (Figure 1a & b). The heart of this technique lies in the coating of droplets with hydrophobic nano-micron-sized particles, commonly referred to as liquid marbles. These particle-coated droplets then encounter oil-infused surfaces, setting the stage for capillary-driven cloaking due to porous network of particles. The presence of particles promotes and stabilizes the formation of an immiscible liquid film around the droplet, effectively encapsulating it (Figure 1c). Thus, this configuration is termed as Liquid marble on oil-infused surfaces (LMOI).
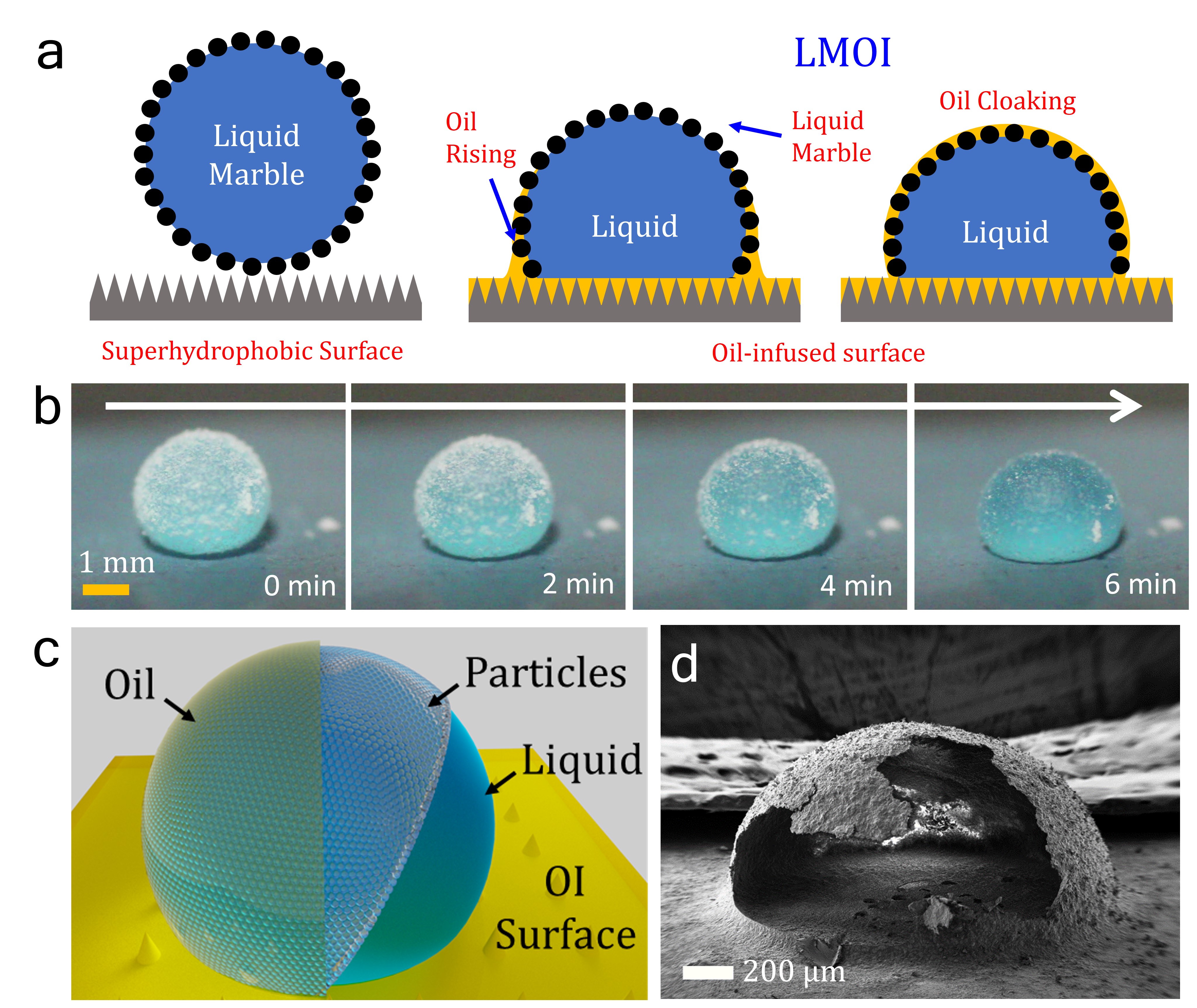
Achieving Unprecedented Tunability:
What sets this approach apart is its exceptional tunability, which extends across four orders of magnitude in droplet volume. We have achieved a remarkable tunability range, spanning from 5 µm to 200 µm, while working with droplets ranging from 14 nL to 200 µL in volume. Liquid shell encapsulation, in particular, offers a means to control the evaporation rate of droplets without the need for complex or specialized equipment. Liquid shell encapsulation extends the lifetime of droplets against evaporation by up to 200 times for tiny 10 µL droplet. Changing the thickness of the liquid coating can be used to tune the evaporation rate of the droplet. This approach achieved an impressive lifetime tunability ranging from a mere 1.5 hours to a staggering 12 days.
Applications Across Scientific Frontiers:
The potential of capillary force-assisted cloaking goes far beyond droplet encapsulation alone. We have demonstrated its utility in single crystal growth for a diverse range of substances, including copper sulfate, Rochelle salt, sodium nitrate, and Lysozyme protein. The feasibility of employing this method for biological applications has been tested successfully, with human and yeast cells thriving in a hanging droplet configuration. Solid capsules, designed to respond to external stimuli such as temperature changes, offer yet another dimension of tunability (Figure 1d). The use of stimuli-responsive oils enables on-demand uncoating and merging, showcasing the versatility and adaptability of this technique.
In conclusion, LMOI, a capillary force-assisted cloaking of droplets, has emerged as a transformative innovation with the potential to reshape the landscape of droplet-based technologies. Its ability to encapsulate droplets of varying sizes, coupled with unmatched tunability in encapsulation thickness and lifetime, positions it as a versatile tool across a multitude of scientific disciplines. From crystal growth to cell culture and beyond, the applications of this groundbreaking technique hold the promise of advancing research and development in diverse fields. As we look to the future, the scalability and versatility of capillary force-assisted cloaking continue to inspire new possibilities, offering a glimpse into the exciting frontiers in droplet-related technologies.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in