Silver nanoparticle-incorporated hydrogel augments the cancer immunotherapy through modulating oral microbiome
Published in Bioengineering & Biotechnology
The oral cavity contains several organs, together with the co-existence of various microbes. Current studies have shown that oral squamous cell carcinoma (OSCC) is accompanied by alterations in the oral microbiome1. However, the role of the microbiome in OSCC is still controversial.
In this research, we sought to explore the hidden role of the oral microbiome in the progression of OSCC. We first detected the difference in microbiota abundance between tumours and paracancer tissues of OSCC. We found that Peptostreptococcus was enriched in OSCC tumor tissues. Interestingly, further analysis found that Peptostreptococcus not only indicated a better prognosis of OSCC patients but also positively correlated with the expression of CD8 in OSCC samples. From this phenomenon, we preliminarily speculated that Peptostreptococcus might improve the prognosis of patients by interacting with the immune system. The further in vivo study showed the tumor growth of mice was indeed significantly inhibited by injecting Peptostreptococcus anaerobius (P. anaerobius) directly into tumors, but not in immuno-deficient BALB/c nude mice. Thus, we proved that the bacteria-mediated immune effect was indeed the main reason for the anticancer effect of P. anaerobius. Then, we investigated the mechanism and found that P. anaerobius could activate DC cells via TLR and NOD pathways.
However, when the salivary microbiota from patients with OSCC was intratumourally injected, the additional injection of P. anaerobius showed no significant therapeutic effect. We speculated that the pre-existence of the oral microbiome prevented the colonization of Peptostreptococcus2. A strategy that can inhibit other microbes might help Peptostreptococcus win the competition with other bacteria. Nanomaterials are known to regulate the behavior, metabolism, and growth of bacterial species. Screening by co-culture, we observed that AgNP significantly inhibited the proliferation of various bacteria except for P. anaerobius. This phenomenon might be attributed to the strong chemical reduction capability of P. anaerobius by reducing the cytotoxic Ag+ to Ag(0), making P. anaerobius more resistant to AgNP.
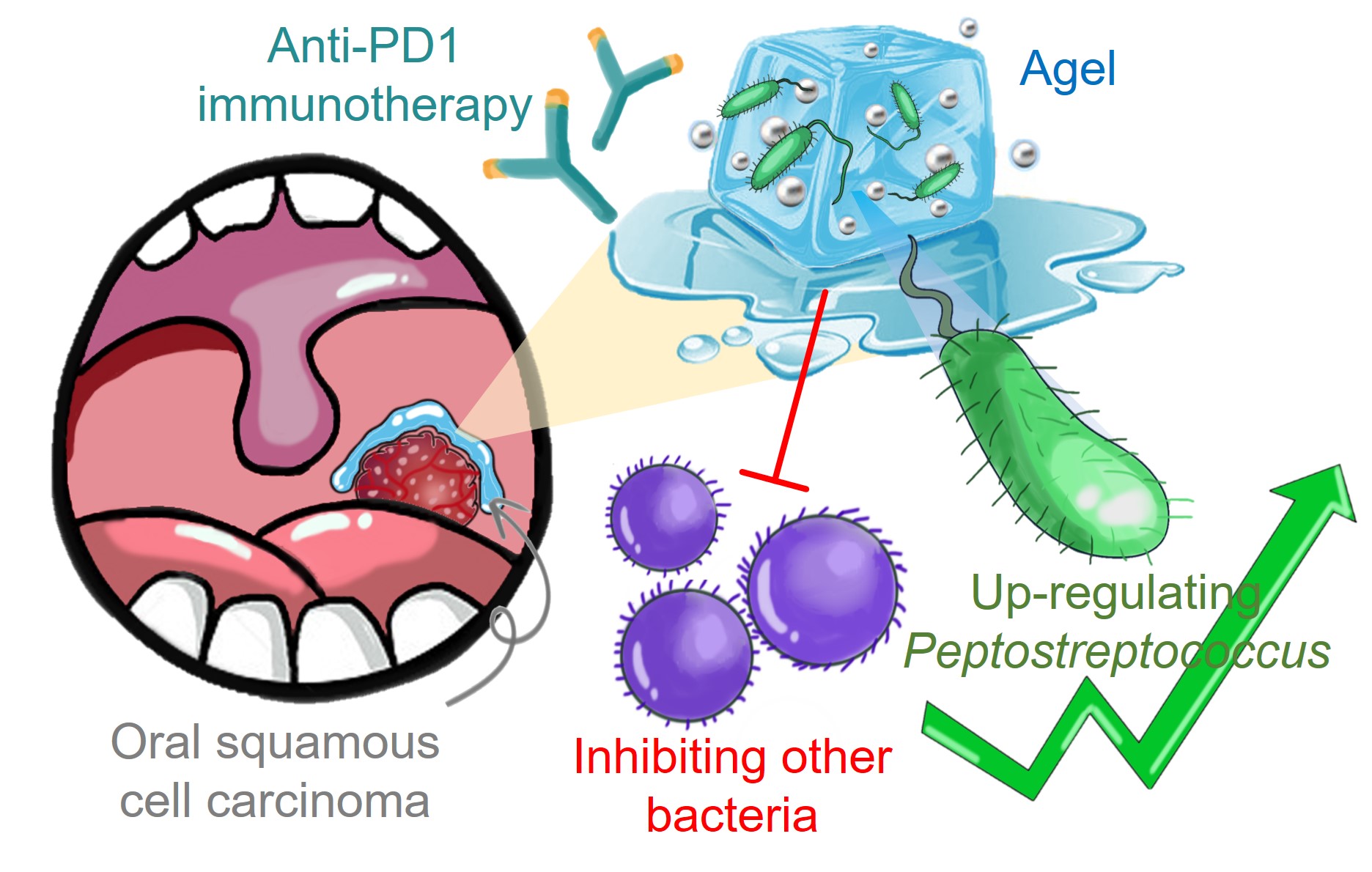
Another problem we face is what kind of administration strategy to choose. Here, we encapsulated AgNP into a hydrogel formulation called Agel that adhered to the oral mucosa3. Agel adhered stably to the oral mucosa for over 24 h. We also proved that Agel induced the DC maturation in draining lymph nodes in 4-NQO induced murine OSCC model and orthotopic 4MOSC1 tumor model.
Immunotherapy, especially for PD-1 targeting therapy, has brought a new dawn for cancer therapy4. Blockade of PD-1 could release the brake of the immune system, and promote the anti-cancer immune response. However, the response rate of the anti-PD-1 antibody (aPD1) is still limited to 14-20% in OSCC. Therefore, exploring the method of enhancing immunotherapy for OSCC is meaningful.
On the basis of this, we successfully explored a strategy for enhancing the efficacy of immunotherapy in OSCC by modulating the oral microbiome. According to the content of Peptostreptococcus, the samples were divided into three groups with high, medium, and low bacterial levels. In mice with oral microbiota with high and middle-level content of Peptostreptococcus, Agel + aPD1 showed tumour-suppressive effects, together with significantly improved intratumoural CD8+ T cells. However, in mice with oral microbiota with low-level content of Peptostreptococcus, only the treatment of Agel + P. anaerobius + aPD1 displayed obvious tumor inhibition. We speculated that treatment with Agel could be applied as a personalized therapy, based on the composition of the oral microbiota. In patients with higher oral Peptostreptococcus content, aPD1 + Agel could be used directly for treatment. In contrast, for patients with lower Peptostreptococcus content, oral microbiota transplantation might be used before aPD1 + Agel administration. We also proved th
e treatment with Agel + P. anaerobius + aPD1 could inhibit the progression of OSCC in 4NQO-based spontaneous model, and various orthotopic tumour models.
In summary, we identified that Peptostreptococcus inhibited the development of OSCC through activating anti-cancer immune responses. Then, Agel with the capacity of modulating oral microbiota was selected to maintain the content of Peptostreptococcus by inhibiting other bacteria species. Importantly, the treatment with Agel and P. anaerobius transplantation may be a personalized therapy based on the content of Peptostreptococcus. Recognizing the close communication between the immune system and the oral microbiota can help us to explore alternative ways for the OSCC treatment, and may provide clinical benefits for patients.
References
- Lamont, R.J., Koo, H. & Hajishengallis, G. The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 16, 745-759 (2018).
- Mullineaux-Sanders, C., Suez, J., Elinav, E. & Frankel, G. Sieving through gut models of colonization resistance. Nat. Microbiol. 3, 132-140 (2018).
- Daly, A.C., Riley, L., Segura, T. & Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 5, 20-43 (2020).
- Ribas, A. & Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350-1355 (2018).
Related content: https://www.nature.com/articles/s41551-021-00807-9
Follow the Topic
-
Nature Biomedical Engineering

This journal aspires to become the most prominent publishing venue in biomedical engineering by bringing together the most important advances in the discipline, enhancing their visibility, and providing overviews of the state of the art in each field.
Related Collections
With Collections, you can get published faster and increase your visibility.
Biosensing
Publishing Model: Hybrid
Deadline: Mar 26, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in