Solution for a long-standing puzzle in polar stratospheric chemistry
Published in Earth & Environment
After the discovery of the ozone hole in 1985, the causes of its formation were discovered quite quickly. Chlorine compounds released from emitted CFCs are activated on cold polar stratospheric clouds, followed by catalytic ozone depletion cycles in which one chlorine atom can destroy many ozone molecules.
Nowadays, the atmospheric models that calculate polar ozone depletion in the stratosphere are able to explain the observed ozone depletion reasonably well. However, based on model-measurement comparison, some details of the chlorine chemistry were not fully understood. The figure below from an earlier publication demonstrates that the satellite observations show a depletion of hydrogen chloride (HCl) over the pole that is not present in the model simulations.
The simulations do not show the HCl depletion in the core of the polar vortex over Antarctica that are visible in the satellite data.
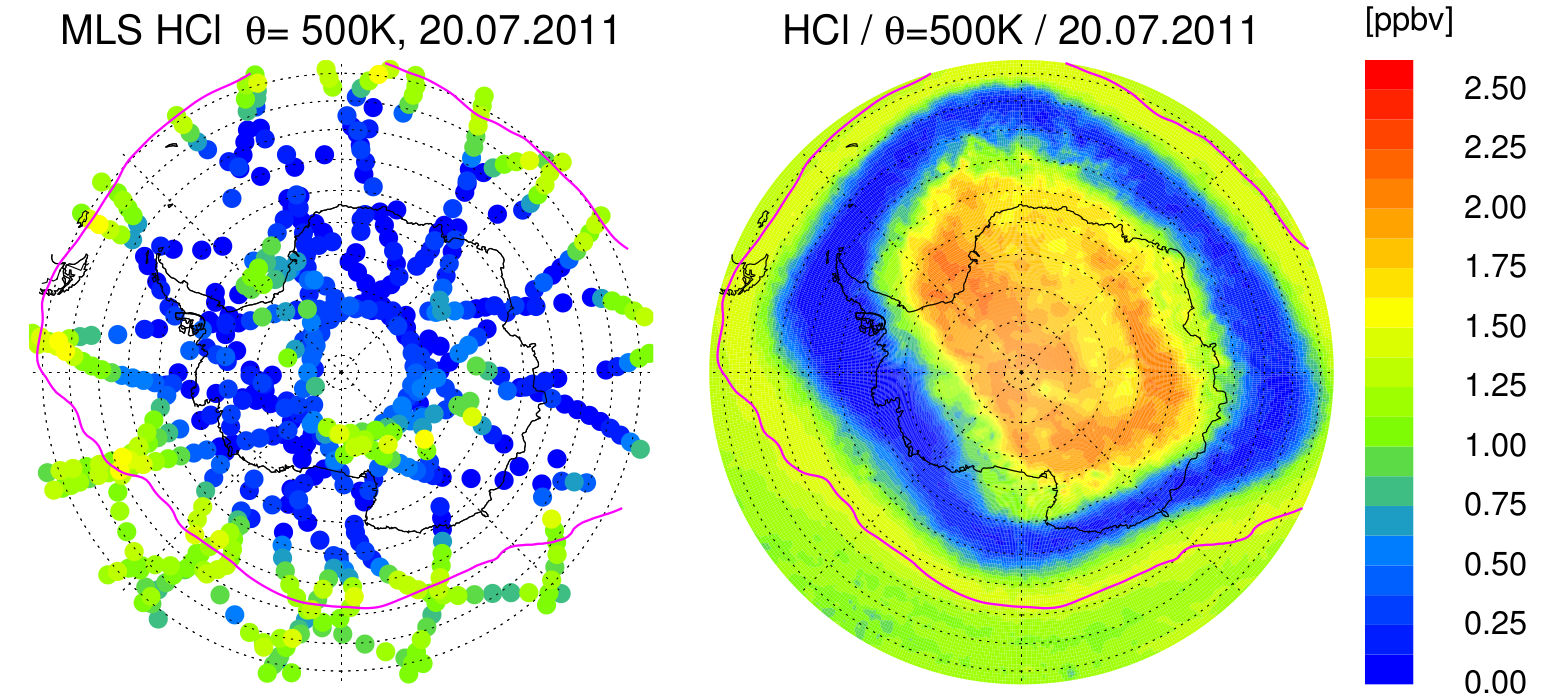
From Grooß et al. (2018)
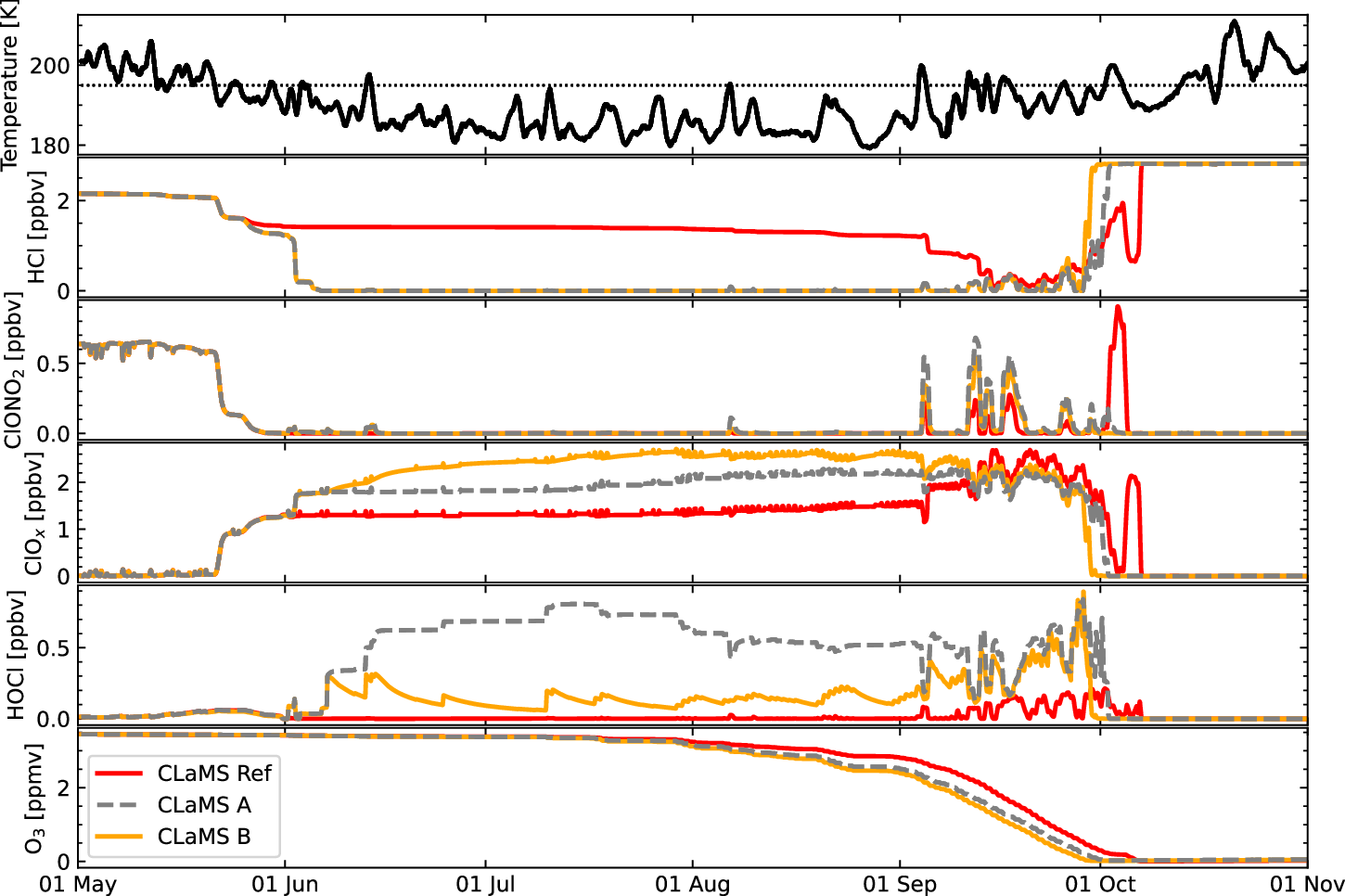
As several models were unable to correctly reproduce the HCl depletion, it appears that a chemical sink was missing, which possibly could have consequences on other chlorine trace gases. There were many hypotheses on what this reaction could be, but no convincing possibility had been found so far. After almost giving up trying to find the missing chemistry, we came across two publications from the year 1997 in which the heterogeneous reaction of chlorine peroxide (Cl2O2) with hydrogen chloride was investigated in a laboratory study with estimation of its impact explored in a simplified box-chemistry model. This study seemed almost being forgotten as it was cited only 4 times since 1997.
This reaction forms gas-phase HOOCl which should be subsequently photo-dissociated likely rather quickly. Already in 1977, before the discovery of the ozone hole, Peter Warneck discussed the potential role of this molecule. However, comprehensive laboratory measurements for the chemical kinetics for the proposed newly added reactions have not been performed so far. In our modelling study, we could show that the heterogeneous reaction between Cl2O2 and HCl is indeed able to deplete the HCl in the polar vortex core as observed. The figure below shows the HCl comparison for the two cases where the reaction was either omitted and included in comparison with satellite data from MLS. Also the comparison with other chlorine compounds ClONO2, ClO and HOCl improves with the new chemistry.
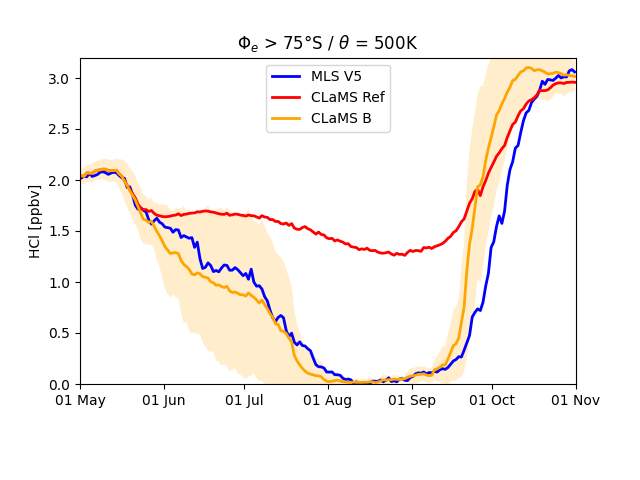
21 km altitude. Red are the previous model results, orange those with the proposed
additional reactions. Corresponding MLS satellite data are shown in blue.
The increase of active chlorine by over 50% in the model causes an increase of ozone depletion of by over 15% between early July and mid September. The simulated ozone depletion is not larger, since the difference in active chlorine occurs within the rather dark polar winter period, but it is still important for the better understanding of the recovery from the ozone hole, that is visible most prominent in September.
As the underlying physical chemistry of the new processes is unknown, we made plausible assumptions concerning rate coefficients and photolysis frequencies. We hope that this publication encourages detailed laboratory measurements of the reaction kinetics of the proposed chemistry.
Follow the Topic
-
Communications Earth & Environment

An open access journal from Nature Portfolio that publishes high-quality research, reviews and commentary in the Earth, environmental and planetary sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Climate extremes and water-food systems
Publishing Model: Open Access
Deadline: May 31, 2026
Archaeology & Environment
Publishing Model: Hybrid
Deadline: Mar 31, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in