Stable p-type carbon nanotubes: toward molecular electronics
Published in Materials
Carbon nanotubes (CNTs) are innovative materials for various devices, including transistors, sensors, photovoltaics, transparent electrodes, and thermoelectric generators, owing to their excellent electrical conductivity, charge-carrier mobility, specific surface area, light-weight, and flexibility. Similar to current silicon-based electronics, effective doping techniques must be developed for CNTs. As electronic devices require p/n junctions, controlling the major carrier type (electron for n-type and hole for p-type) and its density is important for facilitating the intended device functions. Furthermore, the thermal stability of doped states is essential for ensuring long-term operability due to device heating during operation. For instance, logic circuits generate condensed heat owing to the application of electric currents, photovoltaic cells are heated upon exposure to light, and thermoelectric devices work in hot environments.
To overcome this limitation, the Material Physical Chemistry Laboratory of Kobe University (Japan) and the Adhesion and Interfacial Phenomena Research Laboratory of National Institute of Advanced Industrial Science and Technology (Japan) collaboratively developed stabilizing technology of p-doped CNTs. These groups have investigated doping technology for nanoscale carbon materials and conducting polymers, developed characterization technology for quantifying the doping levels, and applied the developed materials to molecular thermoelectric generators.
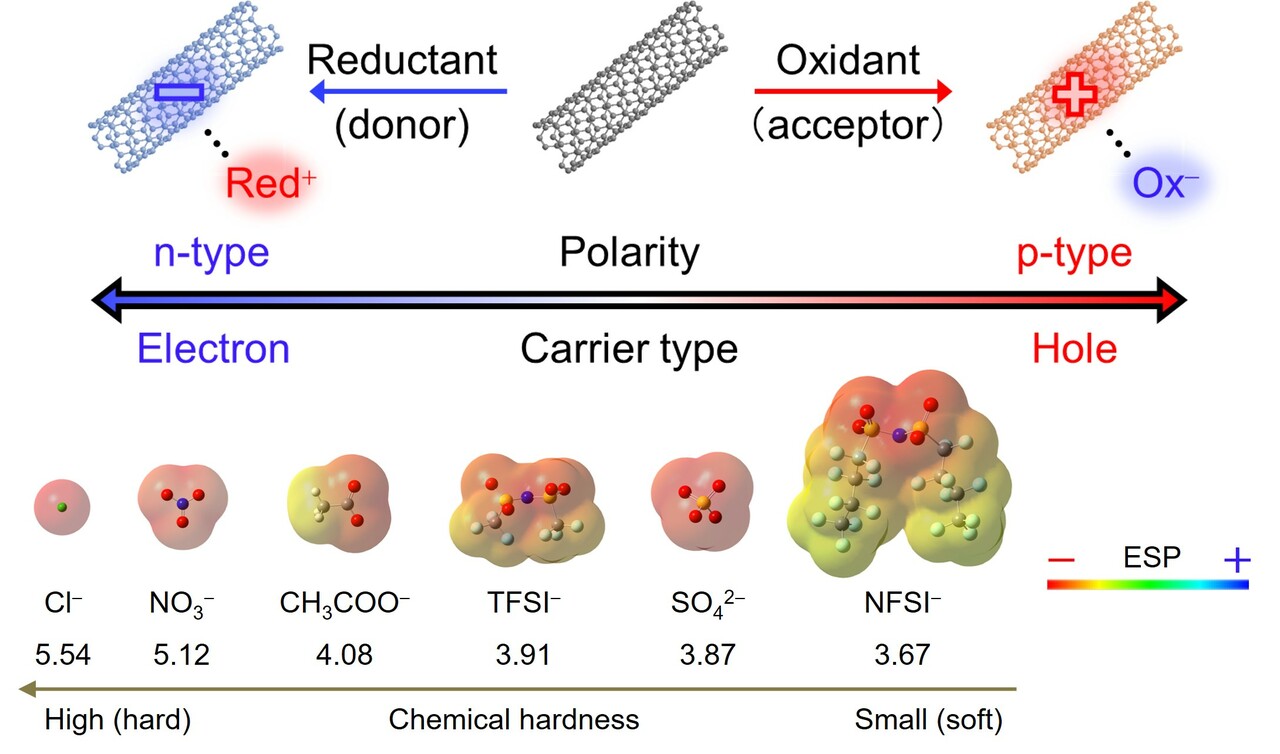
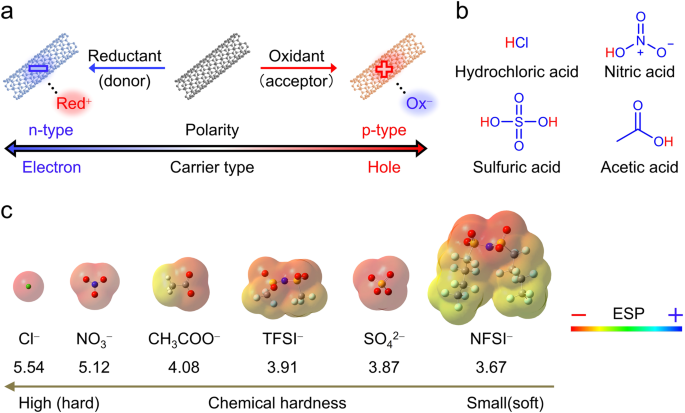
Electrons or holes are injected into CNTs via charge transfer interactions (e.g., π–π, n–π, σ–π, and ion–π interactions) between CNTs and dopants. Reductants (Red) donate electrons, whereas oxidants (Ox) donate holes to CNTs, resulting in n- (anion) and p- (cation) doped CNTs, respectively (Fig. 1). The doped CNTs acquire positive (p-type) or negative (n-type) charges, with counter ions (Red+ or Ox-) adhering to the tube surface to balance these charges (complex formation).
We tried to unveil the underlying stabilization mechanisms of doped CNTs through complex chemistry. The hard and soft acid and base (HSAB) concept is useful for comparing the stability of complexes. Generally, soft acids and bases are large and possess low charge density and high polarizability, and vice versa. Pairs of hard/hard and soft/soft cations and anions produce more stable complexes. As charge carriers injected in CNTs are expected to delocalize through their p-conjugations, the p-doped CNTs are considered soft cations that may stabilize when coordinated with soft anions.
As a quantitative parameter for comparing the hardness of chemical species, chemical hardness was previously introduced as a parameter of the resistance of a chemical species to change its electronic configuration. We employed several protonic acids as model compounds for verifying the HSAB concept in complexes with p-doped CNTs owing to the abundance of anion species (Fig. 2) and the released proton acting as the electron acceptor. Protons efficiently withdraw electrons from CNTs, and subsequently, anions adsorb onto the resulting CNT cations for coordination. Further, these anions possess distinct chemical hardness values (Fig. 2), allowing to compare the effect of hardness on doped CNT stability.
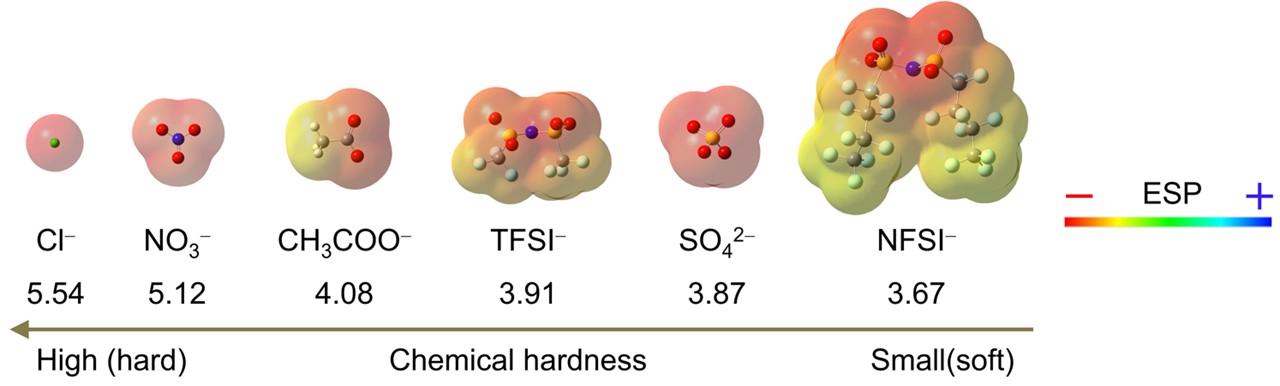
Fig. 2 Molecular structures of the anions used in this study with their electrostatic potential (ESP) mapping as molecular surface, and the order of chemical hardness.
Self-standing CNT (mixed semiconducting (s-) and metallic (m-) tubes with the diameter of 1.6±0.4 nm) films with good electrical conductivity of ~1160 S cm-1 and a Seebeck coefficient of ~+45 µV K-1 were used as the control. Doping could be simply carried out by immersing the film into the aqueous solutions of acids. As shown in Fig. 3, the electrical conductivity drastically increased up to 5680 S cm-1, whereas the Seebeck coefficient decreased to approximately +20 µV K-1 upon doping with the acids. The increase in electrical conductivity and decrease in the Seebeck coefficient indicate hole doping by the acids, reflecting the interdependence between these parameters in relation to carrier density. Further, we found that sp3 defect was not introduced in the tubes during such mild treatments by acids through Raman scattering measurements.
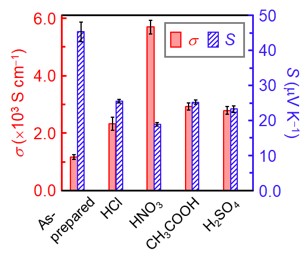
Fig. 3 Variations of electrical conductivity (σ) and Seebeck coefficient (S) of the doped carbon nanotube films according to the applied protonic acids.
To investigate the stability of the p-doped CNT materials, we measured the temporary changes in electrical conductivity and Seebeck coefficient after heating them at 100 °C in air. Figs. 4a-h illustrate the stability of these properties. The thermal stability of the p-doped CNTs in air varies depending on the type of anion. While the electrical properties of H2SO4-doped CNT film remained for extended periods, those of CNT samples doped with HCl, HNO3, and CH3COOH rapidly decreased immediately after incubation commenced. The reduction in electrical conductivity and increase in the Seebeck coefficient indicate dedoping following incubation. As SO42- is considered the softest among the tested anions deriving from protonic acid (Fig. 2), good coordination between the CNT cation and the adsorbed anion is considered responsible for the thermal stability of the doped state.
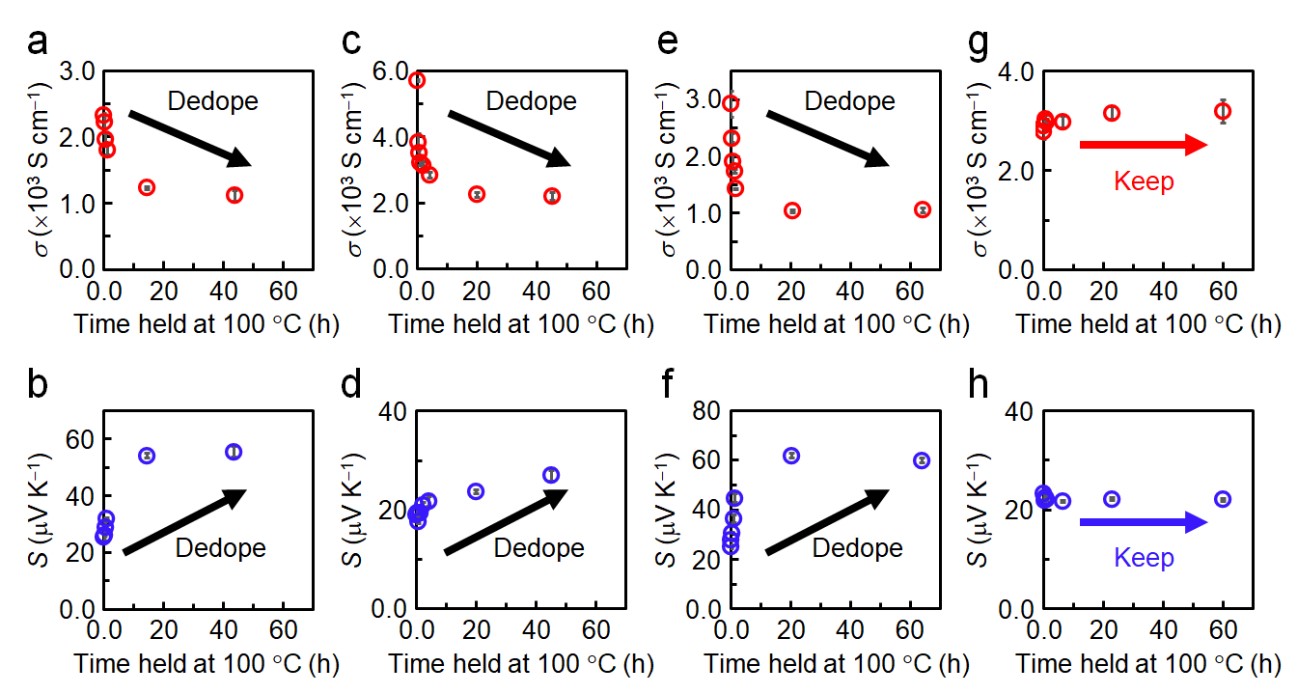
Fig. 4 Temporal changes in the electrical conductivity (σ) and Seebeck coefficient (S) of CNT samples doped with (a,b) HCl, (c,d) HNO3, (e,f) CH3COOH, and (g,h) H2SO4 during incubation at 100 °C in air.
The dedoping process of HCl-, HNO3-, or CH3COOH-treated CNTs upon heating can be attributed to the desorption of the dopants and the disappearance of the injected holes. Suppressing this reaction would lead to the stabilization of the doped states. Specifically, replacing the anions derived from acids with softer anions can enhance the coordination with CNT cations, leading to improved stability. Accordingly, we used several lithium salts comprising bulky, charge-delocalized anions, bis(trifluoromethanesulfonyl)imide (TFSI-), and bis(nonafluorobutanesulfonyl)imide (NFSI-), to stabilize the p-doped state of CNTs (Fig. 5). These anions are chemically softer than Cl-, NO3-, and CH3COO-, with NFSI being even softer than SO42- (Fig. 2).
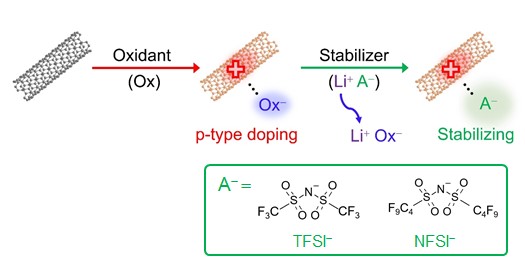
Fig. 5 Schematic of anion replacement for creating stable p-type carbon nanotube complex.
Anion replacement could be performed by simply immersing the p-doped CNT films into the acetone solutions of Li-TFSI or Li-NFSI for only 5 min, and the replacements were confirmed by spectroscopic observations. Figs. 6a,b show temporary changes in the electrical conductivity and Seebeck coefficient of CNT films doped with HNO3 and subsequently treated with Li-TFSI solutions. In contrast to the acid-treated samples, the properties of these films remained stable over 9,000 h (>1 y) at 100 °C in air.
In summary, an understanding of the stability of chemically doped CNTs and its enhancement was achieved through complex chemistry. The coordination between the soft CNT cation and the applied anions was determined to affect the stability of the p-doped states. Replacing the anions originating from protonic acids with soft counterparts lead to the stabilization of the doped states, which endured high temperatures for extended periods. Overall, these findings represent a significant step toward the development of CNT-based devices and the elucidation of transporting phenomena in doped CNT materials through the enhancement of their thermal stability.
Follow the Topic
-
Communications Materials

A selective open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of materials science.
Related Collections
With Collections, you can get published faster and increase your visibility.
Advanced characterizations of high-entropy materials
Publishing Model: Open Access
Deadline: Mar 31, 2026
Fundamental science and applications of silk proteins
Publishing Model: Open Access
Deadline: Mar 31, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in