Synthesis of Unsubstituted Nonacene
Published in Chemistry
Acenes, a class of polycyclic aromatic hydrocarbons consisting of linearly fused benzene rings, have received great attention from not only academic point of view, but also due to their potential uses in various practical applications. Particularly, unsubstituted acenes longer than pentacene are intriguing from both experimental and theoretical point of views; however, the preparation and handling of such acenes have been quite difficult due to their low solubility and high chemical reactivity.
So far, several strategies have been developed to achieve the unsubstituted long acenes. The basic concept is use of soluble and stable precursors to generate the target molecules. The precursors are generally converted to the long acenes at low temperature in dilute conditions in matrices or on surface in ultra-high vacuum. Note that these strategies cannot afford macroscopic amounts of the long acenes, which is necessary for practical applications. In our paper, we have resolved to synthesize nonacene in preparative scale (Figure 1).
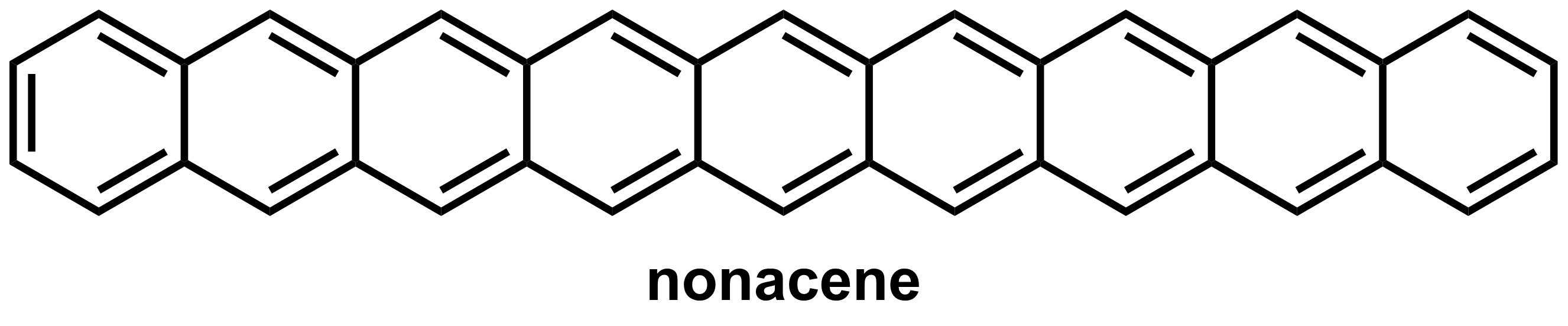
Figure 1. Molecular structure of nonacene
The strategy leading to long acenes is based on a deprotection of suitable carbonyl precursors, which are stable and soluble compounds. After purification using traditional in-solution techniques, are these precursors quantitatively deprotected by heating at moderate temperatures in the solid-state. In our previous work, we have demonstrated that 7,7-dimethoxy-2,3,5,6-tetramethylbicyclo[2,2,1]-heptane (1) can be used in the Diels-Alder reaction with various aromatic arynes to give acene precursors with a dimethyl ketal group1–4 (Figure 2). The ketal moiety is then hydrolyzed to a carbonyl group which can be expelled by thermal or photochemical decarbonylation in solid-state form affording the acenes and carbon monoxide as only by-product.
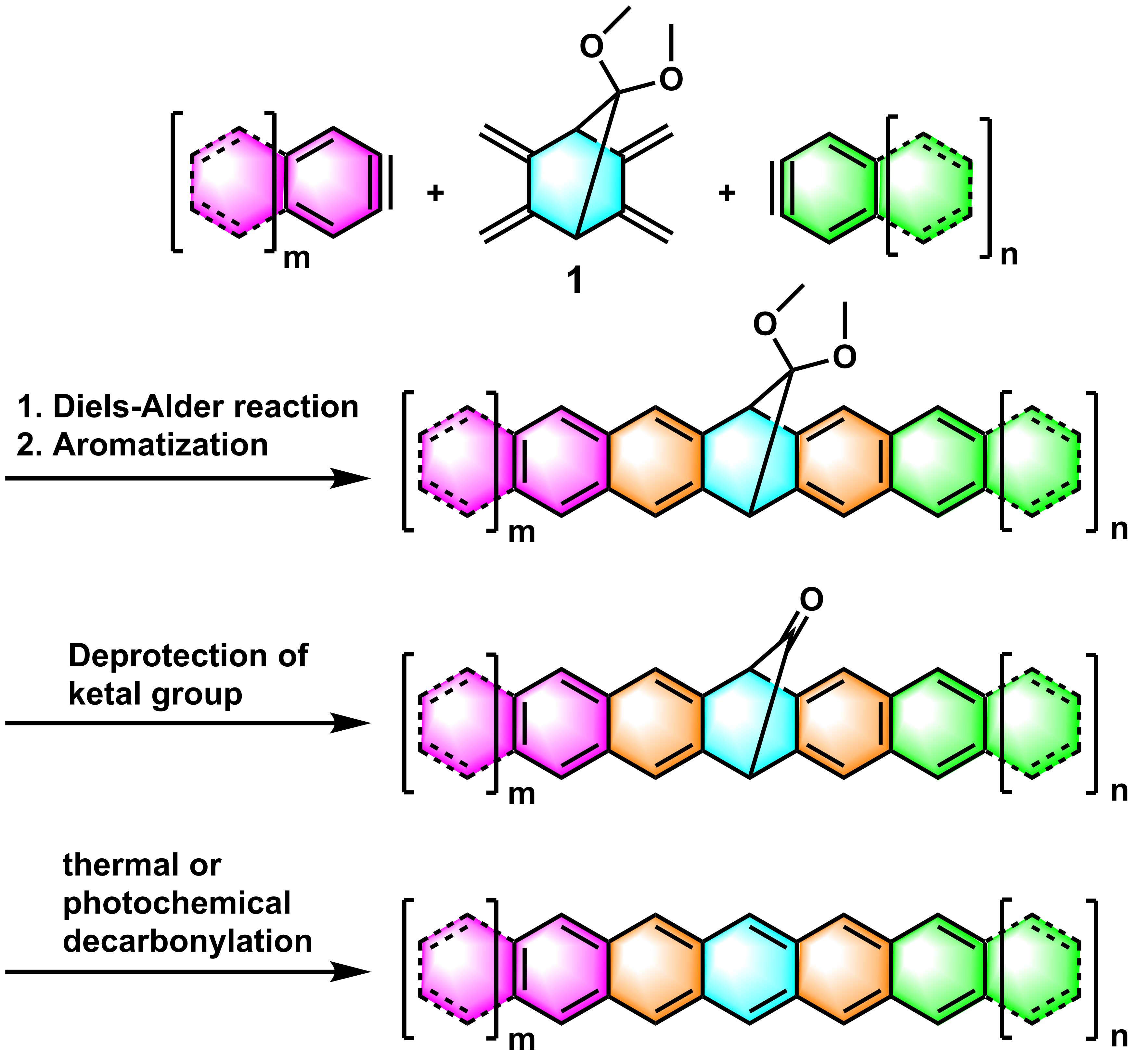
Figure 2. Schematic strategy of the synthesis of acenes starting from 1
Our synthesis of nonacene started (Figure 3) with key double Diels-Alder reaction between diene 2 and bis(aryne) in situ generated by the fluoride-induced decomposition of 2,5-bis(trimethylsilyl)-1,4-phenylene bis(trifluoromethanesulfonate). The double Diels-Alder reaction successfully proceeded in the mixture of solvents (acetonitrile : THF - 4 :1) the desired product with 62%. Here, this product was a mixture of anti and syn isomers in ratio 1:2. The favorable formation of syn-isomer is due to a solvent effect (stabilization of transition state) which was also supported by the density functional theory calculations. The estimated value by the density functional theory calculations (anti/syn = 1/1.64, M06-2X/6-31+G(d,p)//B3LYP/6-31+G(d,p) with the polarizable continuum model (PCM) for acetonitrile) showed a very good agreement with the observed ratio.
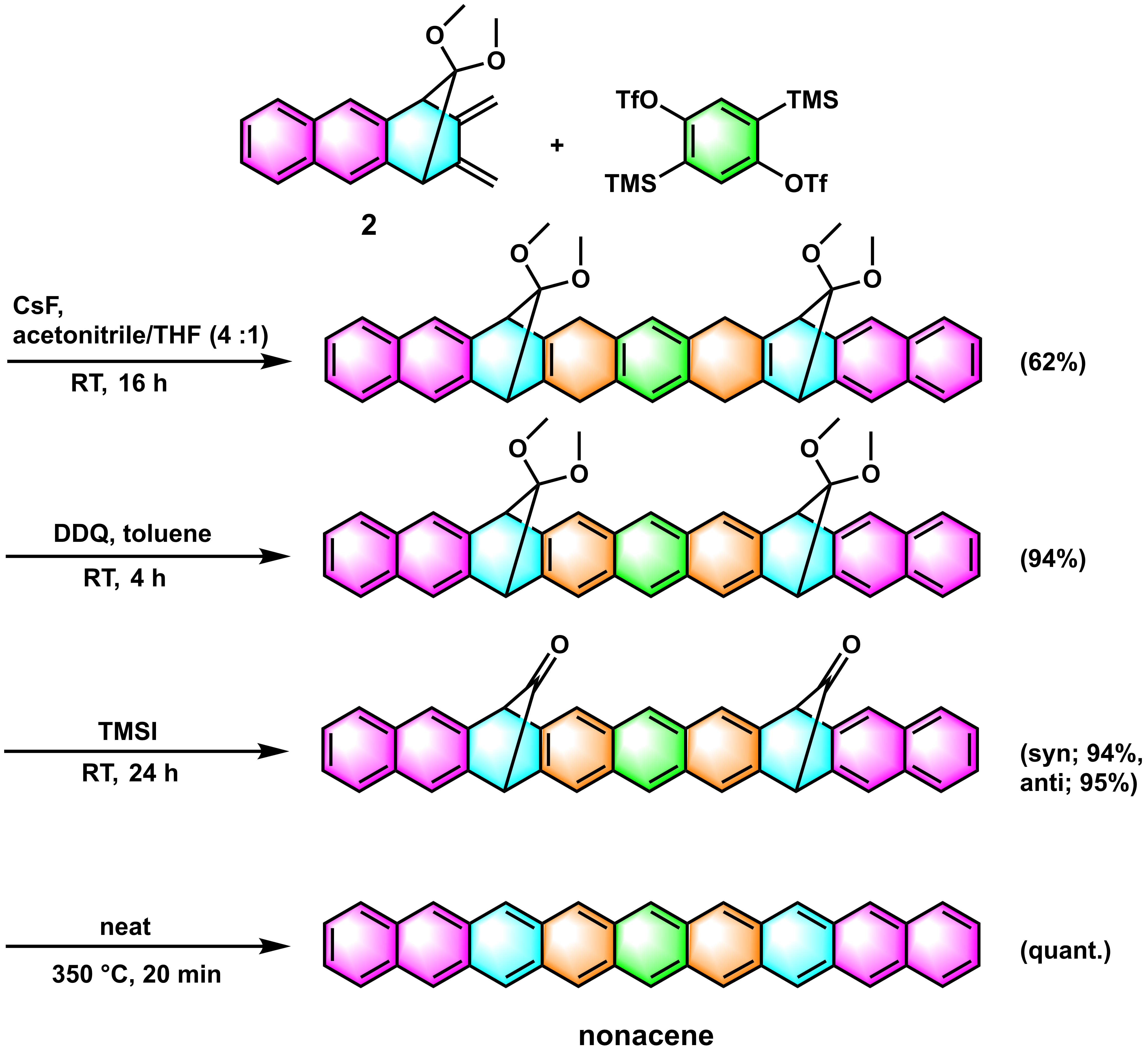
Figure 3. Synthesis of nonacene
The product of the double Diels-Alder reaction smoothly aromatized by 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ). Then the two ketal groups were cleaved by trimethylsilyl iodide (TMSI) in almost quantitative yield. The final decarbonylation reaction, realized in solid state, was confirmed by the thermal gravimetric analysis (TGA), which revealed that the weight loss corresponded to the loss of two carbon monoxides per one precursor molecule. This reaction was also confirmed by the FT-IR measurements. The stretching vibration of carbonyl peak at 1780 cm-1 of the precursor was disappeared after heating, and the spectrum of the obtained product was in a good agreement with the calculated spectrum of nonacene. Furthermore, this transformation was also supported by the cross-polarization magic angle spinning (CP-MAS) NMR spectroscopy. Interestingly, under dry argon atmosphere, the unsubstituted nonacene was found to be stable up to 450 °C based on the TGA measurements and showed no change in the NMR rotor for 2 months at room temperature.
In summary, we have successfully synthesized unsubstituted nonacene by thermal decarbonylation of the corresponding carbonyl precursor in preparative scale. The obtained nonacene was unexpectedly stable under inert atmosphere. We envision that our preparation procedure will be applied for various long acenes, which leads to develop new organic electronics or spintronics devices.
For more information, please read our article in Nature Communications via:
https://www.nature.com/articles/s41467-021-27809-0
References
- Jancarik, A., Levet, G. & Gourdon, A. A Practical General Method for the Preparation of Long Acenes. Chem. - Eur. J. 25, 2366–2374 (2019).
- Holec, J. et al. A Large Starphene Comprising Pentacene Branches. Angew. Chem. Int. Ed. 60, 7752–7758 (2021).
- Jančařík, A. et al. Synthesis and Absorption Properties of Long Acenoacenes. Chem. – Eur. J. 27, 12388–12394 (2021).
- Levet, G. et al. Preparation of a Key Tetraene Precursor for the Synthesis of Long Acenes Eur. J. Org. Chem. 11, 1658–1664 (2020).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in