Theraputic Gas Generation and Delivery Platform
Published in Bioengineering & Biotechnology and Chemistry
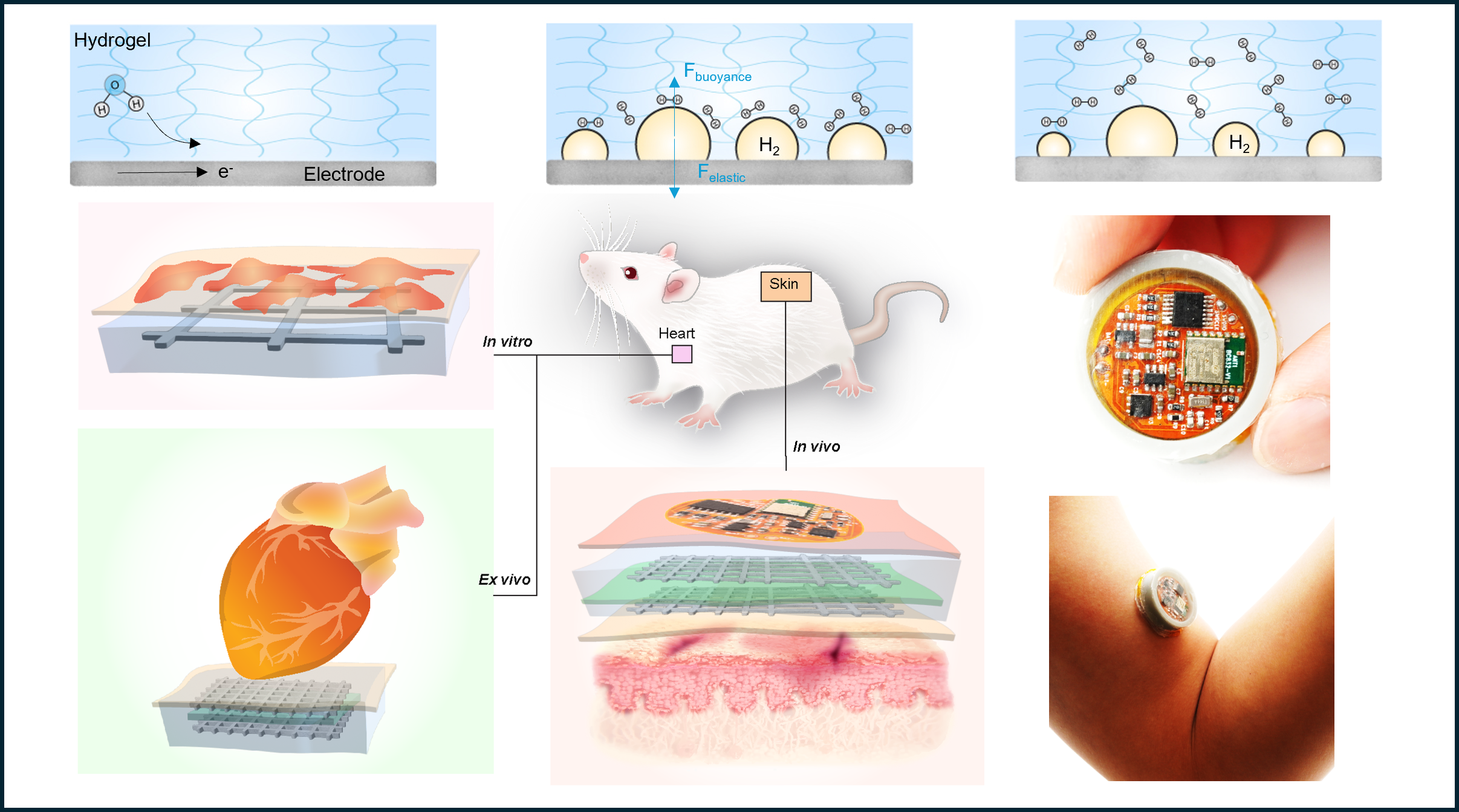
Hydrogen (H2) is the smallest molecule. While it has numerous applications in fuel cells and industrial chemical synthesis and is being explored for energy storage due to its light weight and high energy content upon combustion, one often-overlooked function of H2 is its therapeutic potential.
Our bodies constantly generate reactive oxygen species (ROS). When the body's internal balance is disrupted, these radicals can overwhelm its natural defenses, leading to significant tissue damage. H2 is a fascinating molecule that can address this issue. Because it's so small and light, and generally doesn't interact with most proteins and lipids, it can easily travel and diffuse through tissues. There, it reacts with highly reactive ROS, such as the hydroxyl radical (OH⋅), thereby protecting tissues from further damage.
A major challenge with H2 therapy has been its delivery and controlled generation. Existing methods, like H2 gas inhalation, drinking hydrogen-enriched water, or H2-rich saline injections, often struggle to provide continuous dosing to specific tissues, especially in chronic conditions. The medical doctors we've consulted also raised safety concerns, as H2 is explosive when mixed with air, emphasizing the need for safe handling.
Our Solution: A Hydrogel Electrochemical Cell
In our study, we introduce a hydrogel electrochemical cell designed for the controlled generation, localized storage, and sustained diffusion of H2 under mild, portable, and regulated conditions. Hydrogels are promising materials for bioelectronic interfaces and represent an underexplored system for the hydrogen evolution reaction (HER). As soft materials, hydrogels are highly permeable to gases, making them ideal for gas therapy. In traditional liquid electrolyte-based HER, H2 bubbles escape readily, leading to inefficient diffusion and utilization in biological systems. In contrast, the hydrogel's three-dimensional (3D) water-polymer network supports the HER while effectively and immediately trapping H2 bubbles. This allows for safe and sustainable diffusion and improved delivery at the biointerface. We thoroughly investigated H2 evolution and dynamics within a pure hydrogel electrolyte system compared to a liquid electrolyte system, evaluating how hydrogel polymer composition influences electrochemical kinetics, gas morphologies, and gas storage.
We developed an integrated membrane electrode assembly-hydrogel (MEA-hydrogel) electrochemical device capable of on-demand H2 and O2 production via electrochemical water splitting. This system combines electrochemical gas generation with localized storage and sustained diffusion directly to the targeted tissue, all within a single, portable, and electronically controlled device powered by a flexible printed circuit board.
Validating Therapeutic Outcomes
We validated the therapeutic effectiveness of our device at the cellular level. Using cultured cardiomyocytes and keratinocytes, we demonstrated that our device significantly reduced ROS damage, leading to enhanced cell viability and function under oxidative stress. We further investigated the molecular mechanisms, discovering that our device modulates the NF-κB signaling pathway.
In the final part of our study, we applied our device to animal models. In an isolated ischemia/reperfusion (I/R) heart model, our device effectively reduced myocardial infarction size and restored both electrical rhythm and contractile functions, providing evidences of organ-level protection. We further confirmed the device's therapeutic potential in a clinically relevant skin pressure ulcer model, where it significantly minimized tissue damage by improving metabolic activity, reducing inflammation, and promoting neovascularization.
Future Directions
Beyond H2 therapy, this strategy could be adapted for delivering other bioactive gases, such as oxygen, nitric oxide (NO), and hydrogen sulfide (H2S). Considering that bedsores frequently affect immobile patients during prolonged hospitalization or long-term care, a smart mattress capable of delivering controlled H2 therapy could play a significant role in mitigating ROS damage and enhancing wound healing. Another future direction involves scaling up the device to an electronic therapeutic mattress that integrates H2 sensors with the MEA-hydrogel system, incorporating Proportional-Integral-Derivative (PID) control algorithms for closed-loop, steady H2 supply, and ensuring long-term operational stability.
Follow the Topic
-
Nature Chemical Engineering

This is a new monthly online journal dedicated to publishing the most significant original research, commentary and analysis of direct relevance to the diverse community of chemical engineers.
Your space to connect: The Fuel cell technologies Hub
A new Communities’ space to connect, collaborate, and explore research on Electrochemistry, Chemical Engineering, and Fuel Cells!
Continue reading announcement




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in