
The electrochemical CO2/CO reduction reaction (eCO2RR/eCORR) to value-added chemicals and fuels offers one potential pathway to lower greenhouse gas emission. Substantial progress has recently been made in the performance of the eCO2RR/eCORR system for hydrocarbons below C3. To date, though, longer, valuable, hydrocarbons, C4+, have so far been limited by low selectivity and limited production rates. The factors imply the need for more costly product separation to meet high industrial purity standards for provision of a single C4 isomer.
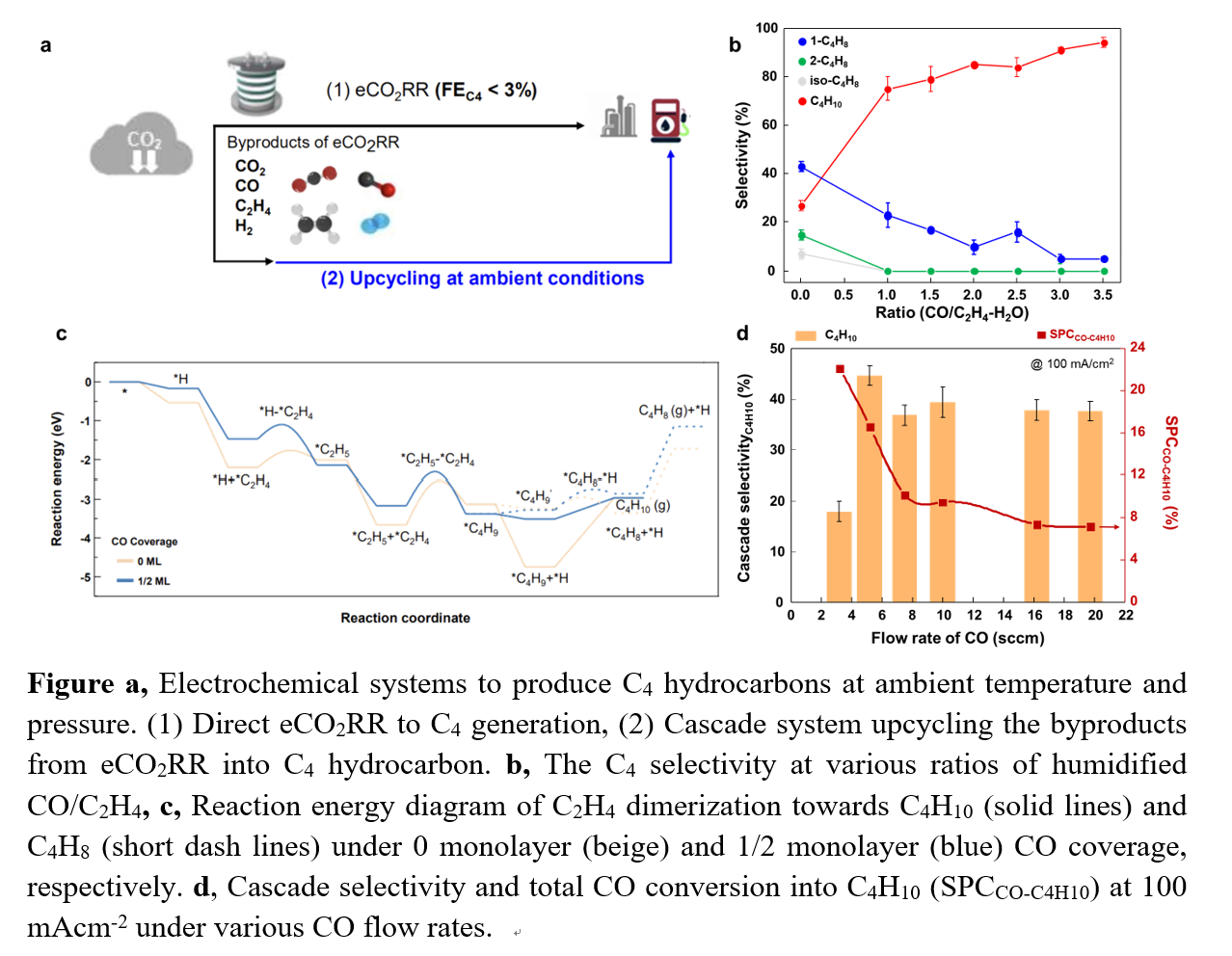
We developed a cascade route that could exploit recent progress in the electrified synthesis of low-carbon-intensity ethylene (C2H4) from C1 (CO2/CO) all the way to C4 hydrocarbons. We pursued a route to feed the outlet of the ethylene system into the dimerization reactor without purification (Figure a), our goal to save both energy and cost.
Cascade systems have been explored that permute electrochemical, thermochemical and biochemical reactions in CO2/CO upgrade to multi-carbon products. However, these systems have often relied on harsh reaction conditions (high temperature and pressure) and generated by-products that necessitate costly separation.
We sought instead a way to selectively produce C4 hydrocarbon under ambient conditions. We pursued the coupled CO2/CO electrolysis with C2H4 dimerization to produce C4 hydrocarbons with high selectivity and C2H4 conversion efficiency under ambient conditions. A direct feed between reactors enabled to reduce separation costs in the system.
To upgrade directly a mixed gaseous stream (CO2, CO, H2 and C2H4) from the eCO2RR stage as input into the C4-producing dimerization reactor, we first investigated the impact of CO2 and CO on modulating C2H4 dimerization to producing C4 hydrocarbons. We found that CO promotes selective hydrogenation during the ensuing C2H4 dimerization unlike CO2. By controlling the CO concentration, we could dramatically improve the butane (C4H10) selectivity to 95% (Figure b).
We performed a density-functional-theory calculation to understand this interesting phenomenon: the relationship between the CO environment and C4 product selectivity. We found that higher CO coverage further destabilizes the *C4H9 intermediate, a factor in initiating the hydrogenation mechanism and steering selectivity from the C4H8 pathway to the C4H10 pathway (Figure c).
Finally, we connected the CO electrolyzer directly to a liquid phase C2H4 dimerization reactor without any purification. As a result, the cascade eCO-to-C4H10 system achieved a cascade selectivity of 43%, C4H10 concentration of 30 wt% and cascade production rate of 24 mM hour-1 to C4H10 under ambient conditions (Figure d).
This example of a combined electrochemical-thermochemical system offers a renewable-electricity-powered pathway for the selective production of C4H10 at ambient conditions while avoiding separation costs between two reactors. The engineered presence of CO may offer a useful degree of freedom in influencing electrochemical and thermochemical reactions to produce higher-molecular-weight hydrocarbons.
If you are interested in our work, you may find the full paper here:
Follow the Topic
-
Nature Catalysis

This journal brings together researchers from across all chemistry and related fields, publishing work on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, incorporating both fundamental and applied studies.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in