What’s Missing in Current Designs for Protonic Ceramic Steam Electrolysis?

Our recent Perspective, titled “Critical insights into the steam electrolysis electrode in protonic ceramic cells for hydrogen production” published in Nature Catalysis, originated from noticing a key oversight in designing anodes—also known as oxygen electrodes—for protonic ceramic electrolysis cells (PCECs). Despite promising efficient hydrogen production at intermediate temperatures, PCECs often borrow successful electrode designs from traditional oxygen-ion-conducting solid oxide electrolysis cells (SOECs), ignoring critical differences—particularly, proton-coupled electron transfer (PCET) mechanisms and the harsh realities of high steam concentrations.
We realized that established strategies from SOECs don’t necessarily apply directly to PCECs. The high steam environments and proton transfer—factors absent in the oxygen electrodes of SOECs—significantly alter oxygen electrode stability and catalytic performance. Our aim was to clearly highlight why SOEC-derived designs frequently fail and to provide guiding principles tailored specifically for PCECs.
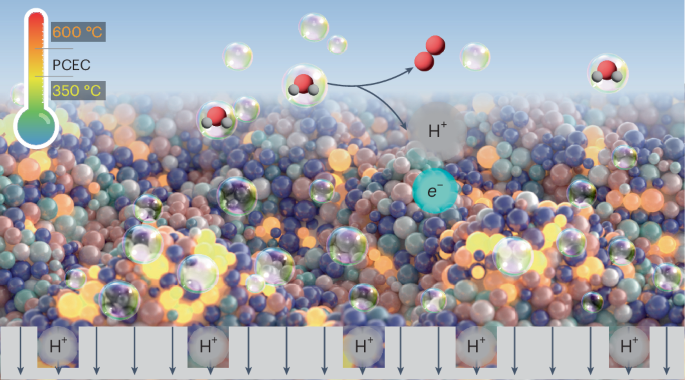
Exploring deeper, we found that traditional catalytic descriptors didn’t adequately capture the unique challenges posed by PCET. For oxygen electrode in PCECs, protons are actively involved in electron transfer, fundamentally altering reaction pathways compared to SOEC environments. This insight highlighted a major gap in understanding electrode stability and catalytic behavior in PCECs. The aggressive steam environments also pose significant challenges to material stability. One primary concern is the increased susceptibility to surface degradation due to the combination of high temperature and steam concentration.
Moving forward, we’re excited to expand on these insights by developing electrodes that can thrive in real-world conditions, leveraging operando characterizations and machine-learning-enhanced simulations. This is just the beginning of a journey that we believe could significantly advance hydrogen technology.
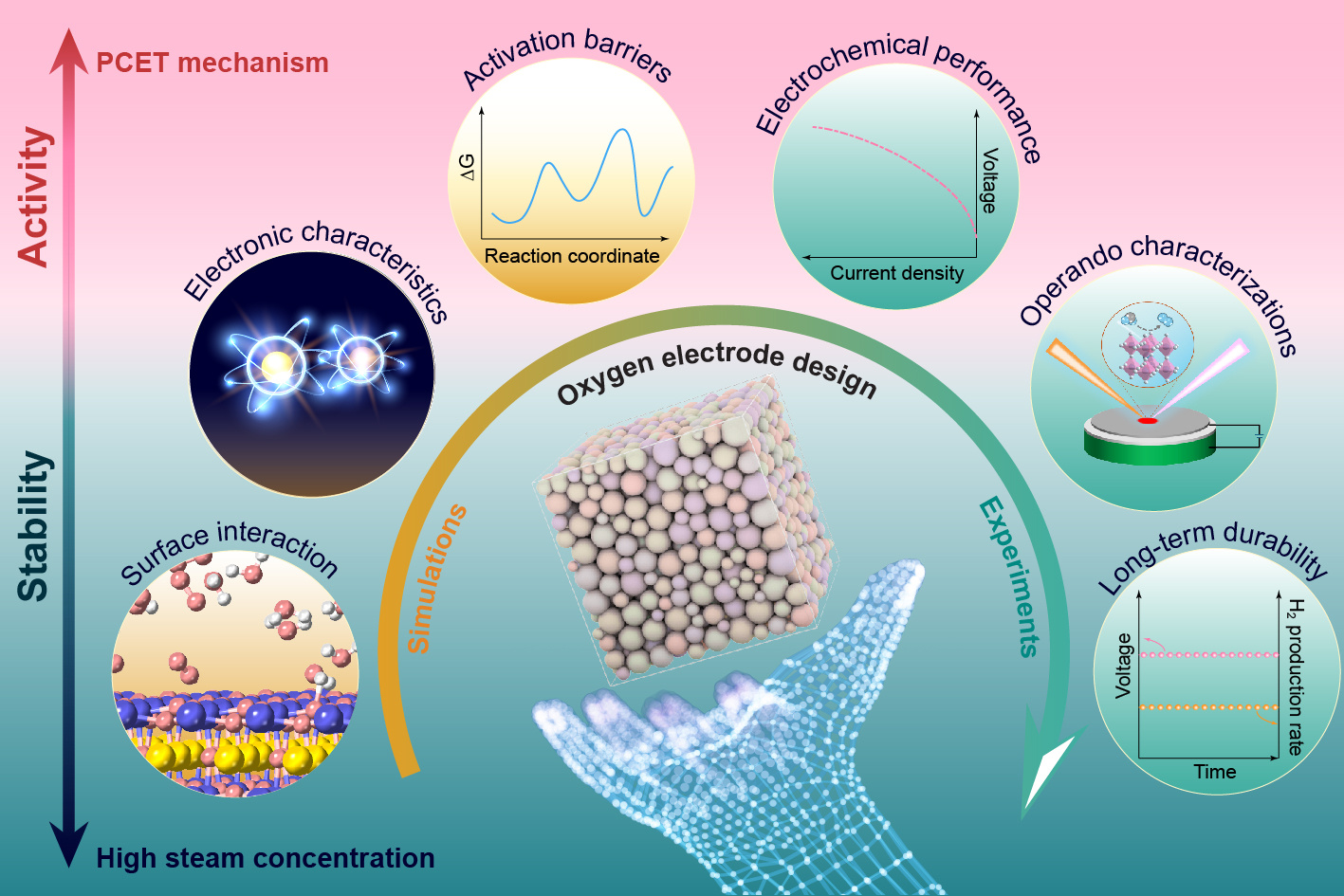
The design principles aim to enhance the catalytic activity of oxygen electrode while maintaining stability under high steam concentrations.
Follow the Topic
-
Nature Catalysis

This journal brings together researchers from across all chemistry and related fields, publishing work on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, incorporating both fundamental and applied studies.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in