The story of plasmonic-catalytic 2D supercrystals for H2 generation
Published in Materials
Investigating plasmonic-catalytic nanomaterials for photocatalysis
In the context of the current world energy crisis, it is essential to develop new materials to replace the role of fossil fuels and decarbonize the industrial processes. Solar light emerges as a promising source of energy and efforts have recently been devoted to harness its potential. For a few years now, physics and chemistry united once more to give rise to the interdisciplinary field of Plasmonic Catalysis. In this field, localized surface plasmon resonances sustained by nanostructured plasmonic metals are exploited to drive chemical reactions. The so-called plasmonic metals (Au, Ag, Cu, Al or Mg) display this optical behaviour within the visible range , allowing them to capture sunlight and channel this energy into molecular bonds. Precisely, this energy is primarily funnelled in the form of excited carriers, as well as local heat.
The increasing research in plasmonic catalysts over the years brought up a considerable gap to fill: in spite of the improved light-driven performances, plasmonic catalysts were still underperforming the traditional catalysts such as Pt, Pd, Ru, among others. Hence, the synergy between plasmonic and catalytic metals in a single structure emerged as a new direction to explore, bringing together optics and heterogeneous catalysis principles to power chemical reactions using light. The combination was proven successful in many cases and for different configurations, but yet the governing working principles of these plasmonic-catalytic nanoscale photoreactors were scarcely understood. These aspects placed plasmonic-catalytic nanoreactors in the centre of our scene, motivating our research in the past years.
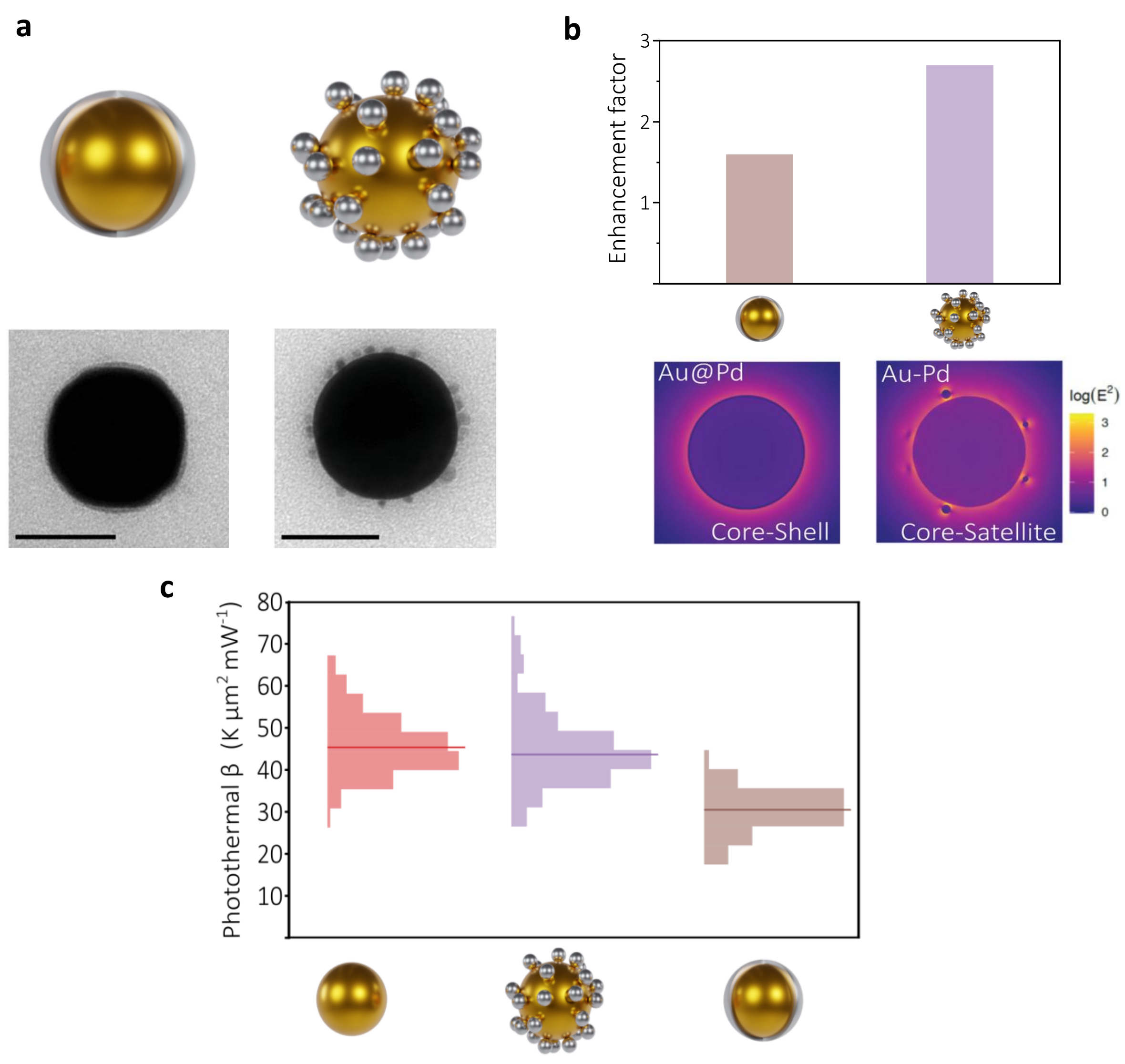
We knew that in its pathway, the energy stored in the plasmon resonance had to flow towards the catalytic active centre to be finally injected in molecules. However, we were aware that the energy flow should be related to the spatial arrangements of the constituents. For instance, when interfaced as in a core@shell structure (plasmonic@catalytic), the plasmonic core can inject excited electrons and holes into the catalytic shell. These excited carriers must travel through the material and finally meet the molecules at the outermost interface. Alternatively, when placed at sub-10 nm distances (antenna-reactor configuration), the catalytic metal can be energized by absorbing light and yield excited carriers right at the active centre. Hence, our primary goal was to elucidate the structure-performance correlation [1] at both the experimental and theoretical fronts. We experimentally observed that antenna-reactor configurations lead to a better performance and, more interestingly, reaction rate's enhancement upon illumination. This boosted performance upon optical excitation was confirmed not only to be because of the larger number of excited carriers within the catalytic metal [2], but also because of the larger temperature field created at the surroundings of the catalytic centre [3]. It was then when we realized that these major effects were consequences of an important structural aspect: the creation of regions with highly confined electric fields between the antenna and the reactor, the so-called optical hotspots. The creation of hotspots have two clear advantages to aid chemical transformations. On one hand, the more intense the electric field, the larger the excited carrier generation rate at the catalytic centre. On the other hand, the lack of an interface between the two metals left the plasmonic resonance almost unaffected, enabling the antenna to deliver energy in the form of local heat to the reactor. Having gained this knowledge at the 'single-particle' level, it was then when we moved forward to tackle one of the major factors limiting the industrialization of plasmonic-catalytic nanoreactors.
From single-catalysts to plasmonic-catalytic 2D supercrystals
One common practice in plasmonic catalysis is to conduct chemical reactions in gas phase by densely supporting the nanostructures in catalyst beds (a cotton or powder). Alternatively, when when performing reactions in the liquid phase, a concentrated solution is used. In both cases, due to the large extinction cross-section, the light penetration into the reactor is limited by photons impinging with catalysts in the first layers. As a result, even when illuminated, some nanocatalyst still operate in conventional thermocatalytic conditions. To circumvent the limitation, we proposed the idea of transitioning from 3D to 2D geometries maintaining the antenna-reactor configuration.
In this regard, 2D plasmonic-catalytic supercrystals were fabricated by a rational control on the surface chemistry of the constituents. Both, the plasmonic (gold, Au) and catalytic nanoparticles (platinum, Pt) were functionalized with thiolated polystyrene and transferred to an organic phase e.g. toluene. By solvent evaporation, the Au nanoparticles were adopting the hexagonal array, incorporating the tiny Pt nanoparticles in the interparticle gap, what enabled to extend the antenna-reactor configuration in two dimensions. Its performance for H2 generation was tested on the well-known formic acid dehydrogenation. This reaction was chosen since formic acid is a carbon-neutral H2 source. Surprisingly, we achieved a two-fold improvement in the performance upon illumination, leading to a world-record activity of 139 H2 mmol gcat-1 h-1 for a plasmonic-catalytic reactor in this reaction [5]. The performance was accomplished with nanograms of Pt, a metal with an inherent worse performance when compared for instance to Palladium (Pd, most active for the reaction).
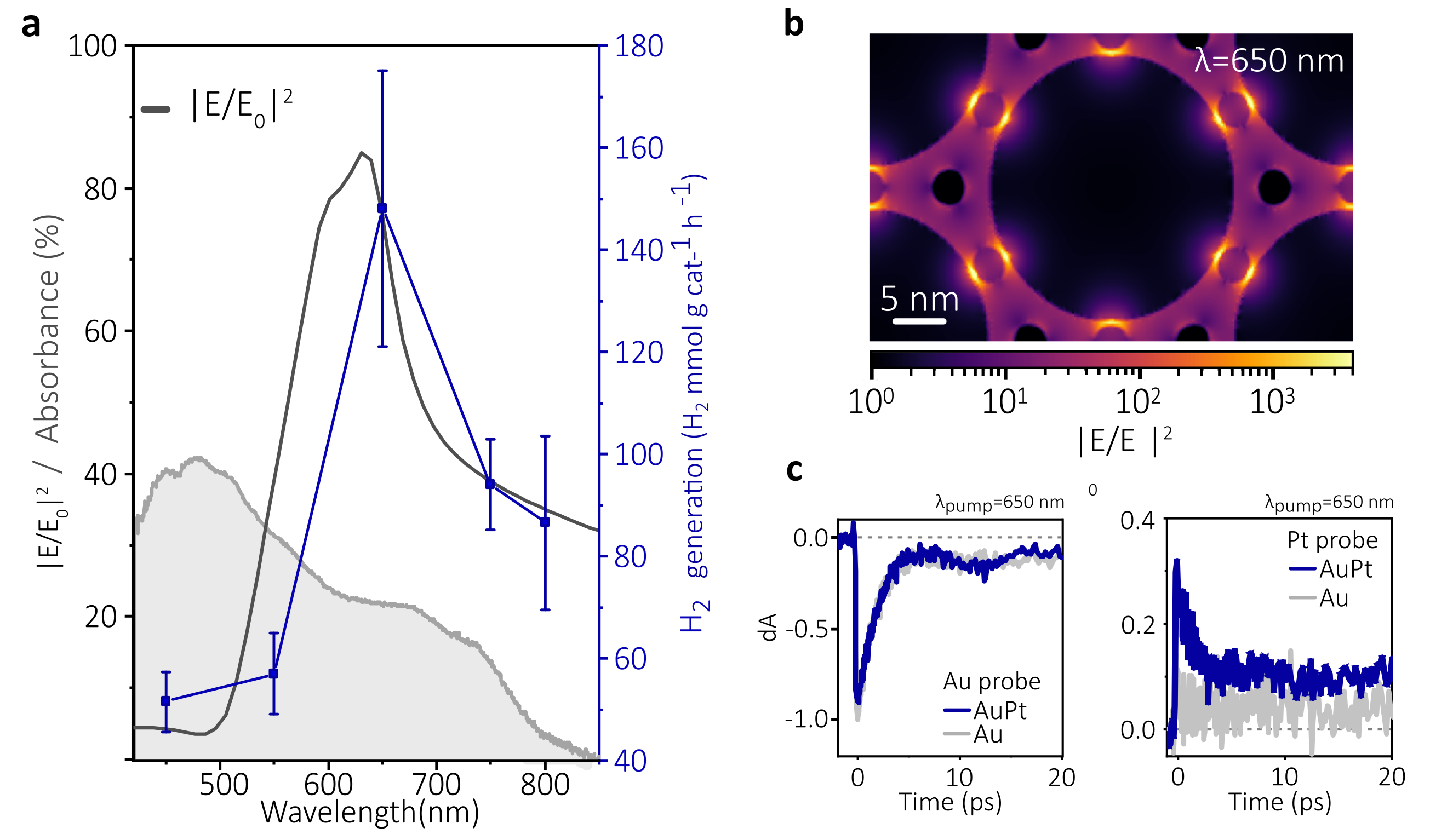
Interestingly, the Au nanoparticles present a collective optical behaviour when arrayed in the 2D supercrystal. This newly optical response, unlike single particles, is characterized by a spectrally decoupled maximum absorbance -taking place in the blue- and electric field enhancement (more intense hotspots) within the green. Hence, we could leverage this decoupled phenomena to go one step further with the underlying physical principles, and unravel which factor was dictating the performance. Hence, through wavelength-dependent experiments and optical simulations, we realized about the strong performance-electric field enhancement correlation, obtaining the best performance when the optical hotspots were more intense. Finally, we carried out ultrafast spectroscopy, which helped us to confirmed the photoexcitation of Pt nanoparticles due to the intense electric field at the hotspots. Therefore, we concluded that t is possible to turn a catalyst with weak visible light absorption into an active sunlight-type photocatalyst via enhanced excitation. Our experiments pointed out that the improved photoactivity is a consequence of the interaction of strongly confined electromagnetic fields (hotspots) with interstitial PtNPs, whereas plasmonically generated heat, appear in comparison to have minimal effect on the reaction rate enhancement.
With the development of 2D plasmonic-catalyic supercrystals, we hope to pave the way towards the incorporation of these types of photocatalysts at industrial level. Among the advantages of the supercrystals, it is worth highlight its straightforward fabrication, as well as incorporation in devices (e.g. photoreactors), and most importantly, the tunability of materials. Future supercrystals should aim for inexpensive materials to replace both the plasmonic constituent (e.g. Copper or Aluminum) and catalytic metals (e.g. Iron).
To finish with, my gratitude goes to all collaborators, who provided valuable input and crucial insight towards the realization of what I personally consider a fantastic piece of work.
References:
[1] Herran, M. et al. Advanced Functional Materials 32.38 (2022): 2203418.
[2] Jin, H. et al. ACS Photonics 2023, 10, 10, 3629–3636
[3] Gargiulo, J. et al. Nature Communications 14.1 (2023): 3813.
Follow the Topic
-
Nature Catalysis

This journal brings together researchers from across all chemistry and related fields, publishing work on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, incorporating both fundamental and applied studies.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in