Visualizing Plant Salt Stress with a NaCl-Responsive Fluorescent Probe
Published in Cell & Molecular Biology
Explore the Research
s41596-024-01068-x?utm_campaign=related_content&utm_source=HEALTH&utm_medium=communities
Salt stress is a major abiotic stress that has a serious adverse effect on plant growth and development. This stress affects plants on >800 million hectares of land around the world, which lead to great losses in agricultural crop productivity. Therefore, procedures for assessment of the levels of salt stress and the consequent management of soil salinity is crucial for alleviating food shortages. At present, the two main techniques used to improve salt tolerance of crops are: transforming the affected soil with plant growth regulators, and cultivating salt-tolerant crop varieties through biotechnology. As a result, an appropriate method to monitor salt stress in plants is of great value because they can be employed to guide implementation of these managements approaches. Moreover, salt stress probes are in high demand in investigations aimed at gaining a fundamental understanding of mechanisms involved in plant responses to salt stress.
Traditional methods for detecting plant salt stress fall into two categories. Invasive techniques, like inductively coupled plasma emission spectroscopy and flame photometry, can quantitatively measure potassium, sodium, or chloride ions but may cause irreversible damage to plants and do not allow real-time monitoring. Non-invasive methods, such as microelectrode ion flux and CoroNa Green fluorescence imaging, offer high sensitivity and rapid detection but have limitations. For instance, microelectrode ion flux quantifies sodium ions via voltage differences on plant tissue but cannot be used on live plants. CoroNa Green is affected by chlorophyll background fluorescence, which reduces detection accuracy. Recently, we developed a novel near-infrared fluorescence probe tool that can visualize and monitor salt stress in plants in situ, in real-time, and in vivo.
Unlike traditional sodium chloride probes, this probe's fluorescence sensing mechanism involves a cationic fluorophore that interacts with sodium chloride, resulting in the probe transforming from monomer to J-aggregates, accompanied by changes in fluorescence intensity (Figure 1a). Compared to CoroNa Green, this probe exhibits bright near-infrared fluorescence, effectively shielding background fluorescence interference from plant chlorophyll, making it particularly suitable for visual tracking of salt stress processes in microscopic samples and living plants, without being affected by extreme conditions like drought or high temperatures. The probe also has a strong targeting function for plant cell mitochondria, allowing for the monitoring of changes in salt concentration within mitochondria under salt stress, providing a powerful tool for studying the molecular mechanisms of salt stress at the subcellular level.
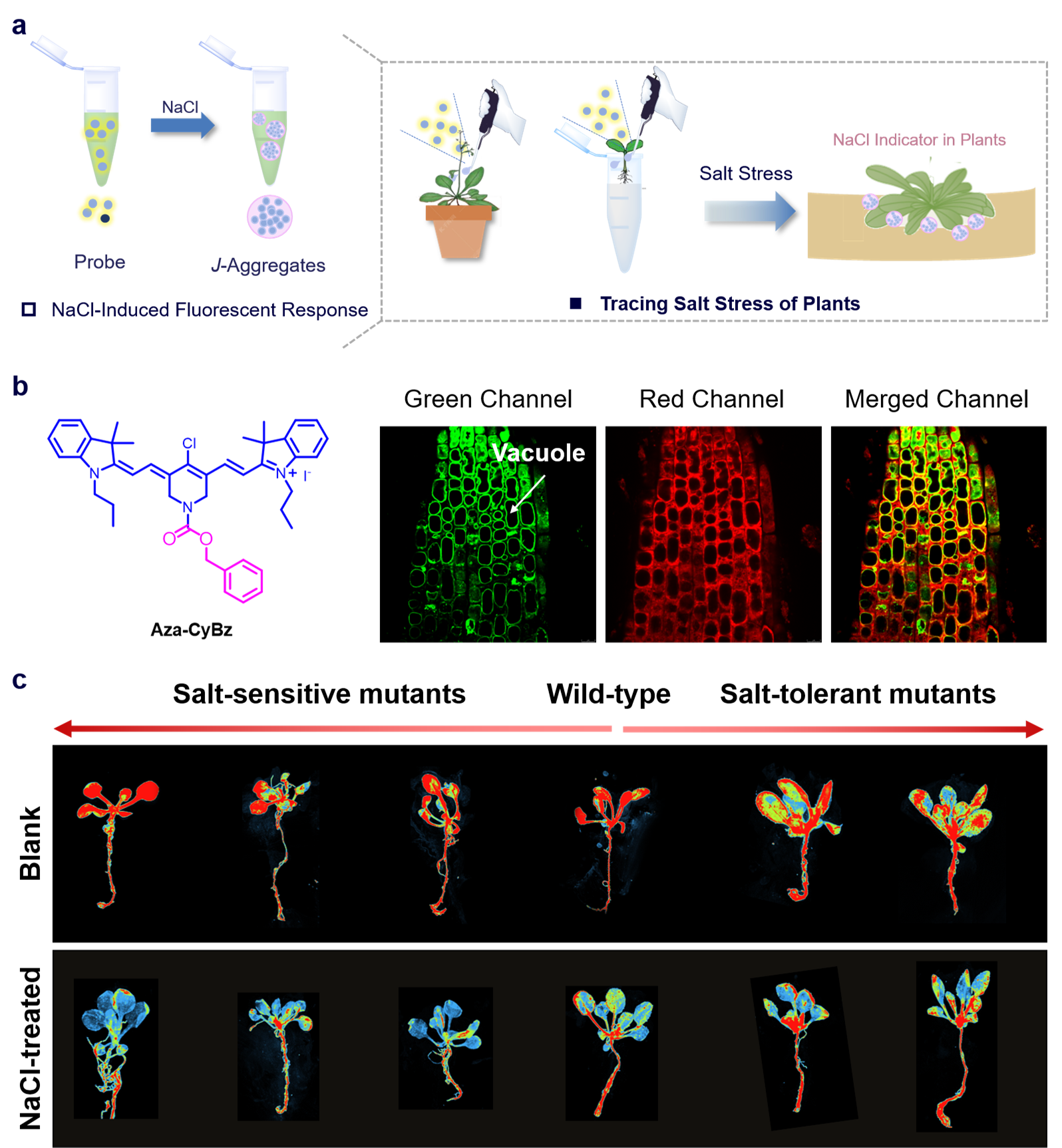
Figure 1: Schematic illustration of visualizing plant salt stress in root tips and live imaging, along with its application in mutant screening.
Typically, when faced with high concentrations of sodium chloride, plants enhance their stress resistance through two main pathways: one is to expel sodium chloride from the cytoplasm, and the other is to sequester salt ions in vacuoles. High concentrations of sodium chloride in the cytoplasm can cause severe damage to plant cells, so assessing the concentration of sodium chloride in the cytoplasm can accurately reflect a plant's stress resistance. The probe developed in this study predominantly distributes in the cytoplasm (Figure 1b), with very low levels in vacuoles, allowing for precise monitoring of dynamic changes in cytoplasmic salt concentrations. It has demonstrated exceptional identification capability in the live screening of salt stress mutants (including sensitive and salt-tolerant types) (Figure 1c).
This study provides a new monitoring method for assessing salt stress in plant root tip tissues and living plants, marking an important breakthrough in the application of molecular imaging technology in agriculture. This technique has significant potential for applications in live fluorescence imaging, plant protection, research on abiotic stress, plant salt tolerance, the molecular mechanisms of salt stress, screening of plant growth regulators, and molecular breeding of salt-tolerant crops.
For more detailed information, see our article " Visualizing plant salt stress with a
NaCl-responsive fluorescent probe" in Nature Protocols (https://doi.org/10.1038/s41596-024-01068-x).
Follow the Topic
-
Nature Protocols

This journal publishes secondary research articles and covers new techniques and technologies, as well as established methods, used in all fields of the biological, chemical and clinical sciences.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in