Spheroids, three-dimensional (3D) clusters of self-assembled cells, are widely used for 3D cell culture system due to their ability to replicate native cell-to-cell and cell-to-matrix interactions similar to living tissues. However, their applications have largely been limited to individual or randomly mixed spheroids, without spatial control over their arrangement. This limitation led us to develop aspiration-assisted bioprinting (AAB), a technique we first introduced in 2020 to enable precise spatial positioning of biologics in 3D (Science advances, 6(10), eaaw5111).
AAB uses controlled aspiration pressure to gently lift and place spheroids at defined locations on a gel substrate or support material to build complex 3D structures. The method supports a wide range of spheroid sizes and cell types and has since been applied in various areas of tissue engineering and disease modeling. It can be used independently or integrated with other bioprinting techniques, such as extrusion-based or droplet-based printing, to form hybrid systems that combine the strengths of each bioprinting strategy for fabricating complex and functional tissues.
To improve scalability, we recently developed an enhanced AAB platform called HITS-Bio (High-throughput Integrated Tissue fabrication System for Bioprinting) (Nature communications, 15(1), 10083). This system features a digitally controlled nozzle array, with each nozzle operating independently to enable the simultaneous and precise placement of multiple spheroids. The high-throughput capability expands the potential for fabricating larger and more intricate tissue constructs efficiently.
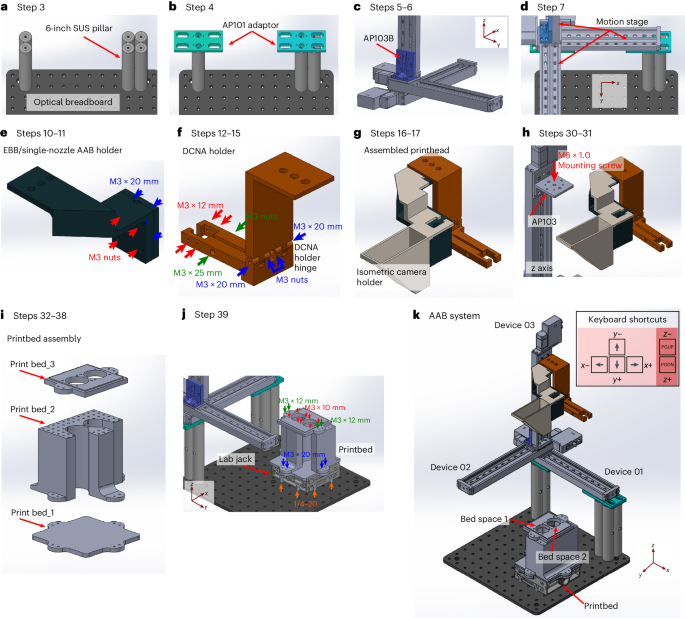
In this Protocol paper, we present a detailed step-by-step guide to building and operating the AAB system. This protocol reflects our aim to make bioprinting more accessible, customizable, and reproducible. It includes comprehensive instructions for hardware assembly, software setup, optimization of bioprinting parameters, and key operational procedures.
We describe two primary modes of AAB operation: (1) single-nozzle mode and (2) high-throughput mode. The single-nozzle mode (Fig. 1a) offers greater flexibility and is ideal for experiments requiring high spatial precision and sequential placement of different spheroid types, such as studies of multicellular interactions. The high-throughput mode (Fig. 1b), in contrast, utilizes the nozzle array to control the placement of multiple spheroids simultaneously, enabling rapid and scalable bioprinting well-suited for large tissue constructs and for intraoperative bioprinting use in surgical settings (Fig. 1c).
To assist future users, we include practical tips and troubleshooting guidance based on years of hands-on experience, from system assembly to operation in both modes. The platform is efficient and user-friendly, and researchers can assemble a fully functional AAB system in about a week. Once operational, it supports precise, reproducible bioprinting adaptable to both small-scale and large-scale applications.
Looking ahead, we believe AAB will continue to evolve and expand its applications through adoption and innovation by the scientific community via this Protocol. We hope it empowers researchers to explore new applications of AAB and contributes to advancing the field of engineered living systems.
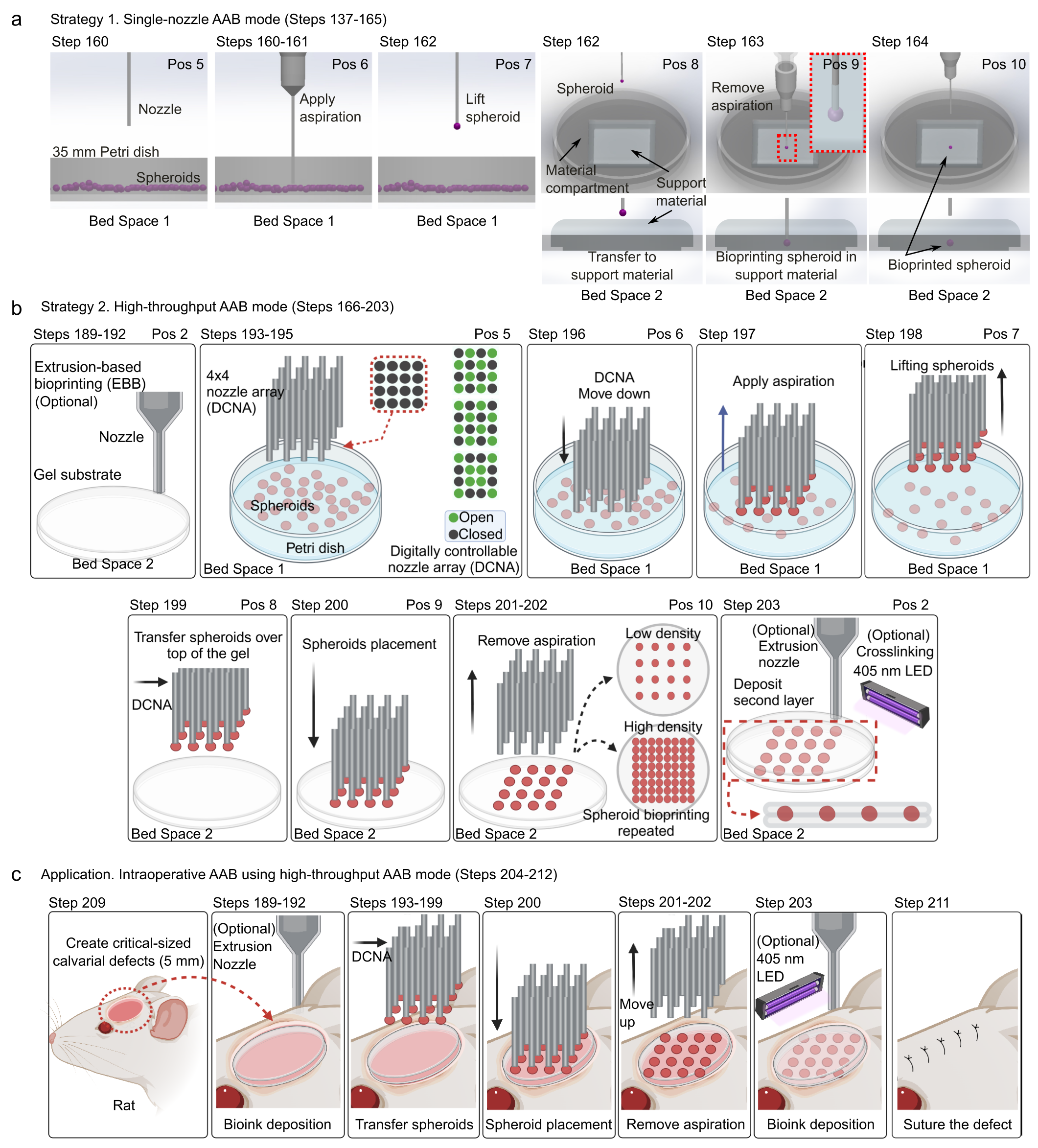 Fig. 1. a,b, A schematic illustration detailing the step-by-step procedure for the AAB process in single-nozzle AAB (a) or high-throughput AAB mode (b). c, An intraoperative application of the high-throughput AAB mode for calvarial defect regeneration.
Fig. 1. a,b, A schematic illustration detailing the step-by-step procedure for the AAB process in single-nozzle AAB (a) or high-throughput AAB mode (b). c, An intraoperative application of the high-throughput AAB mode for calvarial defect regeneration.
Follow the Topic
-
Nature Protocols

This journal publishes secondary research articles and covers new techniques and technologies, as well as established methods, used in all fields of the biological, chemical and clinical sciences.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in