Salt-Mediated Synthesis: A Critical Route toward Noble Metal Aerogels
Published in Chemistry

Noble metal aerogels (NMAs) were discovered in 2009, which are a class of emerging porous materials entirely composed of nanostructured noble metals. Although they have found immense potential in catalysis, sensing, plasmonic technologies, and energy conversion applications, their on-target synthesis remains challenging because of the big difference between the sol-gel process of metals and conventional systems. In fact, too much effort has been put into optimizing application performance before having an insightful knowledge of composition/structure control, which may account for the slow development of this field at the first decade.
Conventional synthesis methods for NMAs often suffer from 1) an expensive and complex ultracentrifugation process to yield concentrated NP precursors, 2) the weak ability for structure control (even without the ability to control the ligament size), and 3) a long sol-gel time (several days to several weeks).
Salt-Mediated Protocol: A Series of Versatile Strategies
The described protocol focuses on gaining an insightful understanding of the roles played by various salts (common inorganic salts and reductive salts) in the sol-gel process of metal systems. On this basis, efficient strategies are developed for controlled, effective, and fast synthesis of a wide range of NMAs, laying the foundation for the design of such young materials. Typically, the fabrication process is presented in Figure 1, where salts can either serve as initiators or reductants depending on their chemical nature.
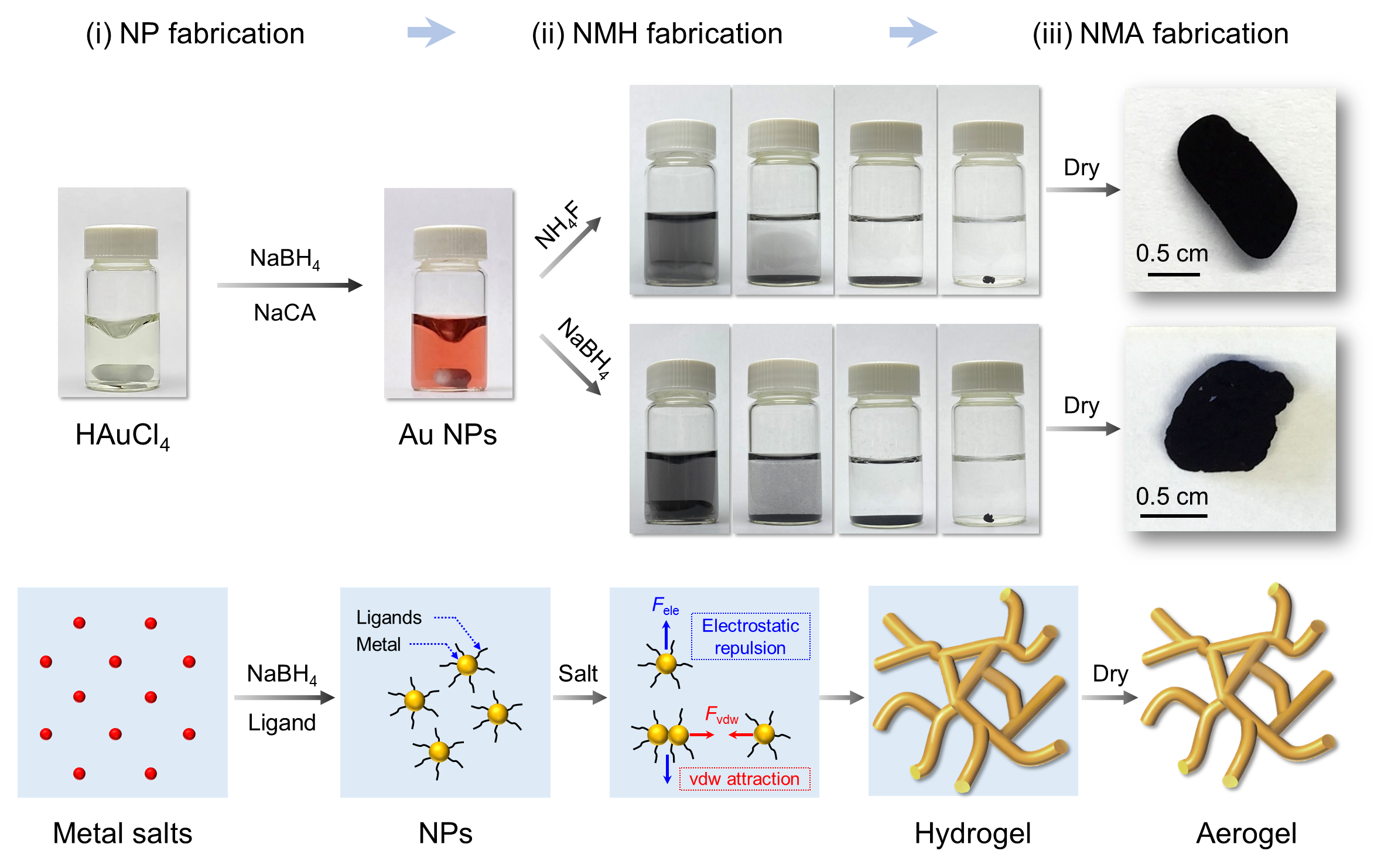
Figure 1 NMA synthesis procedure and the underlying mechanisms. The procedures include (i) the formation of colloidal NPs, (ii) the assembly of the NPs into a hydrogel, and (iii) drying to yield the aerogel.
Ligament Size Tunability (from sub-5 nm to >100 nm)
Our salt-mediated protocol tunes the ligament size of the gels via two approaches, i.e., by 1) applying specific common salts (Figures 2a-d) and 2) combining excessive NaBH4 and specific ligands (Figures 2e-g). The size modulation also adapts to other single- and multi-metallic systems, though not as prominently as the Au system.
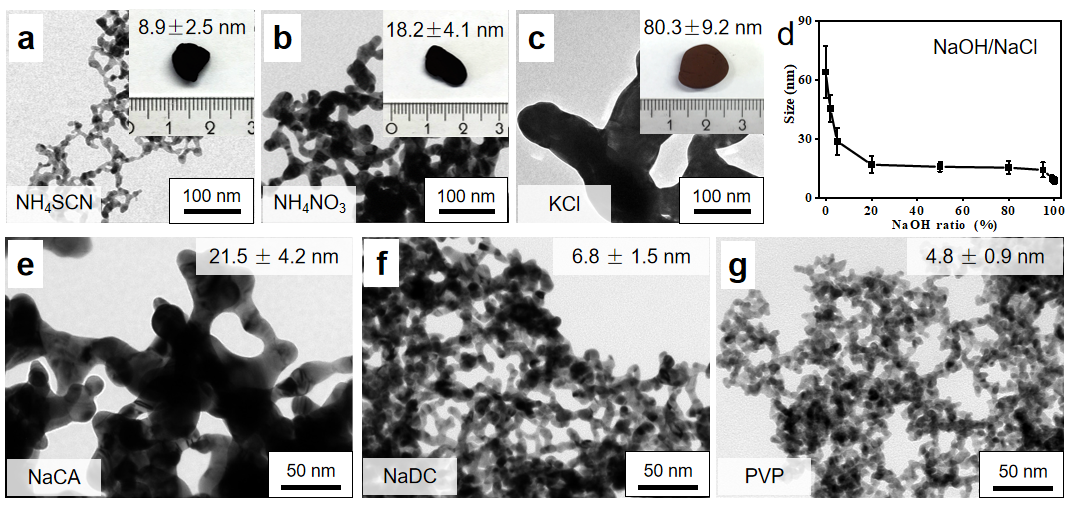
Figure 2 Control of the ligament size of Au aerogels.
Compositional Diversity
NaBH4 as a reductive salt represents an exceptional destabilization ability, enabling the expansion of the available composition of NMAs to single-component Ru, Rh, and Os aerogels and over ten multi-component noble metal aerogels. The method has also been extended to many non-noble metal aerogels in the last few years. Moreover, in combination with an adequate feeding style (we called the dynamic shelling approach), various multimetallic core-shell gels can be easily obtained during the sol-gel process, paving the way to construct complex-structured NMAs with specific functions.
Accelerated Gelation (minutes vs. weeks)
Introducing stirring or other disturbances during the early reaction stage enhances mass transfer, reducing gelation time from days to minutes while retaining the controllability of the ligament size and element distribution (Figure 3). This also benefited from the unique self-healing property of noble metal hydrogels, enabling the formation of a monolithic gel materials by assembling disturbance-led gel pieces.
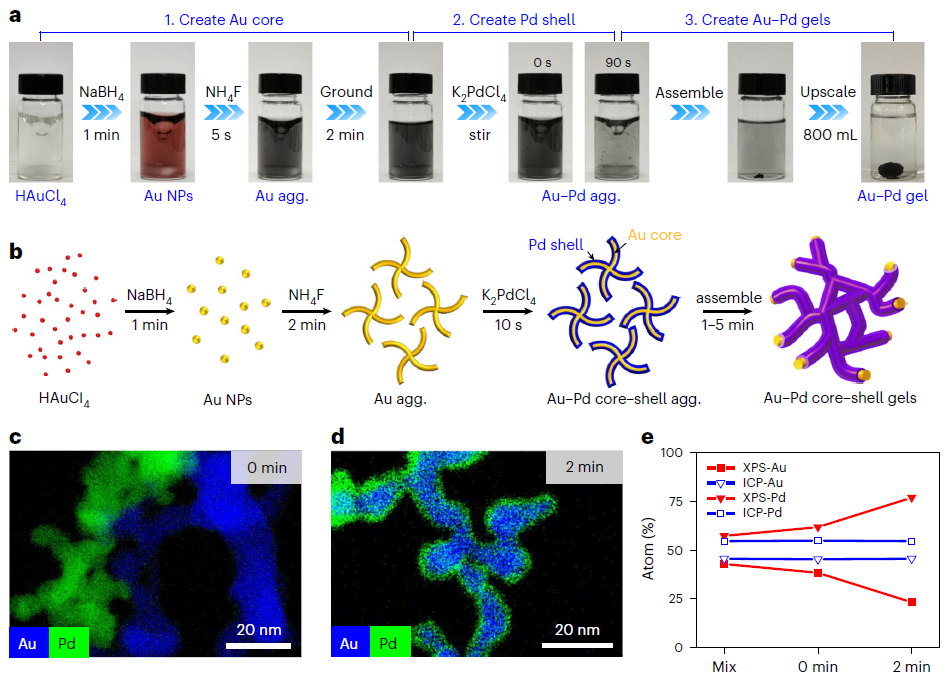
Figure 3 Rapid fabrication of Au-Pd aerogels with controlled element distribution.
Applications
Electrocatalysis: we exemplify the exceptional electrocatalytic performance of NMAs by using the ethanol oxidation reaction, oxygen evolution reaction, and hydrogen evolution reaction. The ideal combination of nano-sized metals as well as aerogel architecture endow materials with both high catalytic activity and high stability.
Conclusion
The salt-mediated protocol described here provides a robust and general method to synthesize NMAs with various compositions, widely adjustable ligament sizes, and controlled element distribution at ambient temperatures. The realization of composition/structure control and expansion for NMAs will promote the communication between nanomaterials and macromaterials, which benefits the understanding of the nucleation, growth, and assembly process of NBBs and enables to bring fascinating nano-effects into the macroworld.
References
- Du, R. et al. Specific Ion Effects Directed Noble Metal Aerogels: Versatile Manipulation for Electrocatalysis and Beyond. Adv. 5, eaaw4590, doi:10.1126/sciadv.aaw4590 (2019).
- Du, R. et al. Unveiling Reductant Chemistry in Fabricating Noble Metal Aerogels for Superior Oxygen Evolution and Ethanol Oxidation. Commun. 11, 1590, doi:10.1038/s41467-020-15391-w (2020).
- Du, R. et al. Disturbance-Promoted Unconventional and Rapid Fabrication of Self-Healable Noble Metal Gels for (Photo-)Electrocatalysis. Matter 2, 908-920, doi:10.1016/j.matt.2020.01.002 (2020).
- Du, R. et al. Rapid synthesis of gold-palladium core-shell aerogels for selective and robust electrochemical CO2 J. Mater. Chem. A 9, 17189-17197, doi:10.1039/D1TA03103A (2021).
Follow the Topic
-
Nature Protocols

This journal publishes secondary research articles and covers new techniques and technologies, as well as established methods, used in all fields of the biological, chemical and clinical sciences.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in