A contractile injection system is required for developmentally regulated cell death in Streptomyces coelicolor
Published in Microbiology
Bacteriophages (or “phages”) are viruses that infect bacteria and are thought to be the most abundant biological entity on earth. Phage sequences present in bacterial genomes have been adapted and repurposed by bacteria throughout evolution to fulfil beneficial roles in bacterial survival and proliferation. Contractile phage tail-like gene clusters, absent the head genes, are found in many species across all bacterial phyla and are now recognized as important mechanisms for intra- and inter-species competition, communication and remarkably even interactions with eukaryotic organisms. Two prominent examples of such systems are the type VI secretion system (T6SS) and the extracellular contractile injection system (eCIS). Despite both systems being discovered around the same time and the fact that eCIS clusters are found across a wider range of microorganisms (including Gram-positive bacteria and Archaea) (1,2), much less is known about the biological roles of eCIS in bacteria.
In this study, we have initially looked at a diverse collection of eCIS genomic sequences identified within hundreds of bacterial genomes, curated in an in-house database in the Davidson lab. What was immediately obvious in our original analysis was the conservation of eCIS-encoding clusters in Streptomyces genomes. Up to that point, eCIS were only known to contribute to interactions between bacteria and eukaryotic organisms, where eCIS were shown to deliver toxic cargo to target cells (3,4). Therefore, the presence of eCIS-encoding clusters in the majority of the soil-dwelling, nonpathogenic, Streptomyces was intriguing. We were also lucky to have our collaborators, the world class Streptomyces lab of Dr. Justin Nodwell, just down the hall.
In experiments using Streptomyces coelicolor (Sco) as a model, we did not observe any direct eCIS-dependent inhibitory activity against bacteria or eukaryotes. We did however detect robust eCIS gene expression and eCIS phage tail-like particle production as part of the normal growth cycle of Sco in standard lab conditions. Mutations in key eCIS genes caused changes in colony development, including changes in antibiotic production. This led us to hypothesize that eCIS may affect intra-colony interactions and that their activity is important for the inherent developmental processes in Sco.
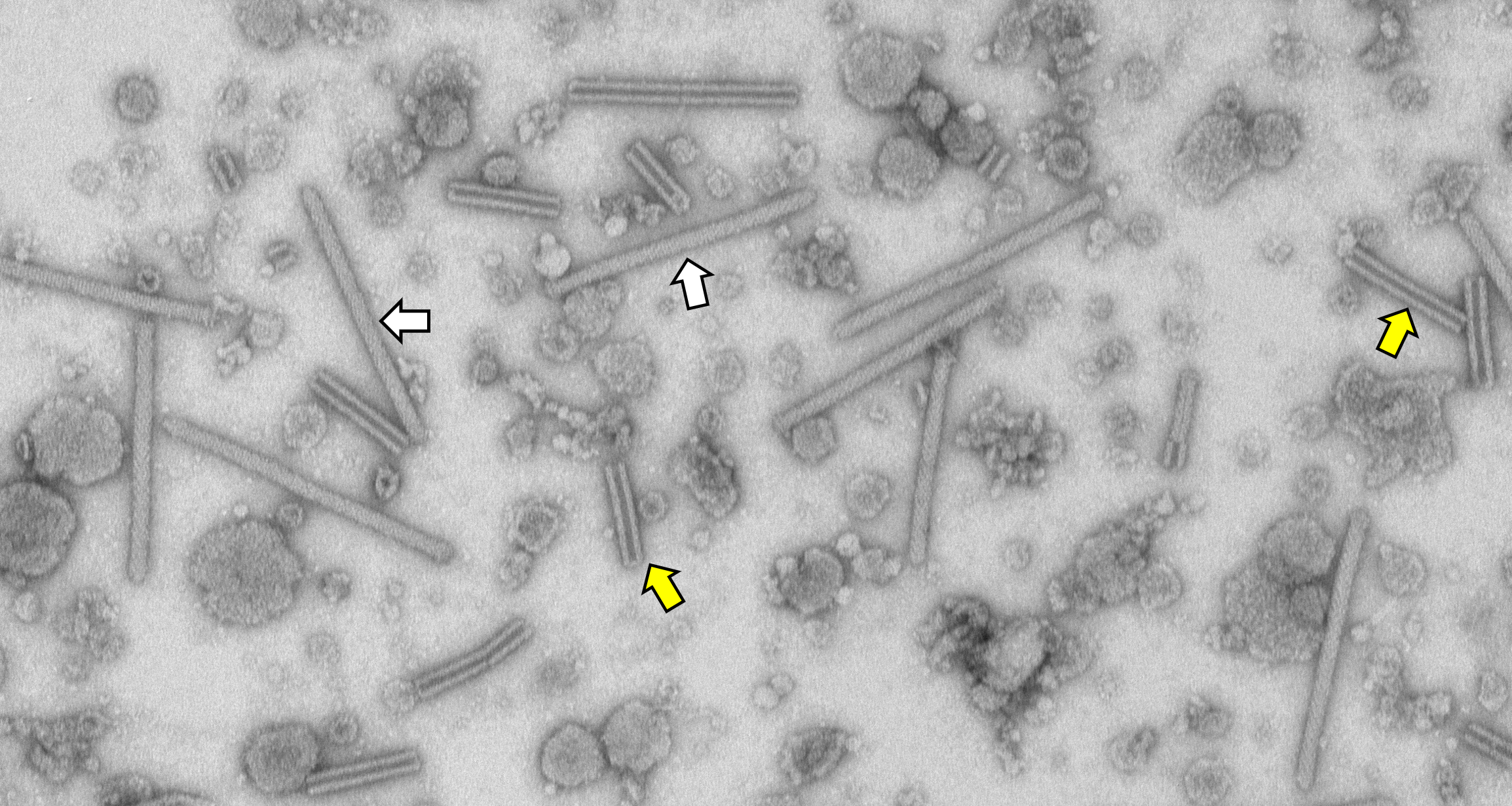
Streptomyces coelicolor produces intracellular eCIS phage tail-like structures. White arrows show examples of mature, extended WT eCIS. Yellow arrows show examples of empty contracted sheaths.
Streptomyces grow as complex multicellular-like vegetative hyphae. Following nutrient depravation or stress, these bacteria undergo a developmental switch, which results in formation of a “reproductive” hyphal subpopulation that matures into spores. As part of this natural process, a large part of the vegetative colony dies, presumably to supply nutrients and support the developing spores. The view of such bacterial processes as “programmed cell death” has been a controversial one, but has now been recognized and described in detail in several species (5).
In this work, we have shown that compared to WT Sco, eCIS deficient mutants exhibited significantly lower levels of cell death and had altered colony morphology during the developmental switch in liquid cultures. These results were also corroborated by a recent study by Casu et al. (6). Despite the disheartening feeling of discovering that another group intends to publish on the same subject, I have also felt a great sense of relief. The fact that a completely independent group observed very similar results in eCIS deficient strains considerably strengthens the validity of our findings and would hopefully encourage more studies into the versatile and fascinating biological roles of eCIS.
Overall, our results suggest that Sco eCIS function by inducing intra-strain lethality, which may play an important role in the developmental process. Additionally, we have predicted that putative pore-forming toxins may act as eCIS effectors. These effectors are encoded in many eCIS clusters of diverse filamentous bacteria, including Actinobacteria, Cyanobacteria, and Chloroflexi. Therefore, the proposed eCIS-dependent mechanism can be common among many bacteria with a complex life cycle.
References
- Chen, L. et al. Genome-wide Identification and Characterization of a Superfamily of Bacterial Extracellular Contractile Injection Systems. Cell Rep 29, 511-521 e512, doi:10.1016/j.celrep.2019.08.096 (2019).
- Geller, A. M. et al. The extracellular contractile injection system is enriched in environmental microbes and associates with numerous toxins. Nature communications 12, 3743, doi:10.1038/s41467-021-23777-7 (2021).
- Hurst, M. R., Glare, T. R. & Jackson, T. A. Cloning Serratia entomophila antifeeding genes--a putative defective prophage active against the grass grub Costelytra zealandica. J Bacteriol 186, 5116-5128, doi:10.1128/JB.186.15.5116-5128.2004 (2004).
- Yang, G., Dowling, A. J., Gerike, U., ffrench-Constant, R. H. & Waterfield, N. R. Photorhabdus virulence cassettes confer injectable insecticidal activity against the wax moth. J Bacteriol 188, 2254-2261, doi:10.1128/JB.188.6.2254-2261.2006 (2006).
- Allocati N, Masulli M, Di Ilio C, De Laurenzi V. Die for the community: an overview of programmed cell death in bacteria. Cell Death Dis. 6(1), e1609, doi: 10.1038/cddis.2014.570. (2015).
- Casu, B., Sallmen, J.W., Schlimpert, S. et al.Cytoplasmic contractile injection systems mediate cell death in Streptomyces. Nat Microbiol. (2023).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in