Behind the Paper: How a microbial metabolite reshapes host branched-chain amino acid metabolism
Published in Chemistry, Microbiology, and Cell & Molecular Biology
 Branched-chain amino acids (BCAAs)—leucine, isoleucine and valine—are essential nutrients that play central roles in protein synthesis and energy metabolism. At the same time, persistently elevated circulating BCAA levels are increasingly recognized as metabolic risk factors, closely associated with obesity, insulin resistance and type 2 diabetes. While the gut microbiota has long been known to influence host metabolism, how microbes regulate host intrinsic BCAA metabolism has remained poorly understood.
Branched-chain amino acids (BCAAs)—leucine, isoleucine and valine—are essential nutrients that play central roles in protein synthesis and energy metabolism. At the same time, persistently elevated circulating BCAA levels are increasingly recognized as metabolic risk factors, closely associated with obesity, insulin resistance and type 2 diabetes. While the gut microbiota has long been known to influence host metabolism, how microbes regulate host intrinsic BCAA metabolism has remained poorly understood.
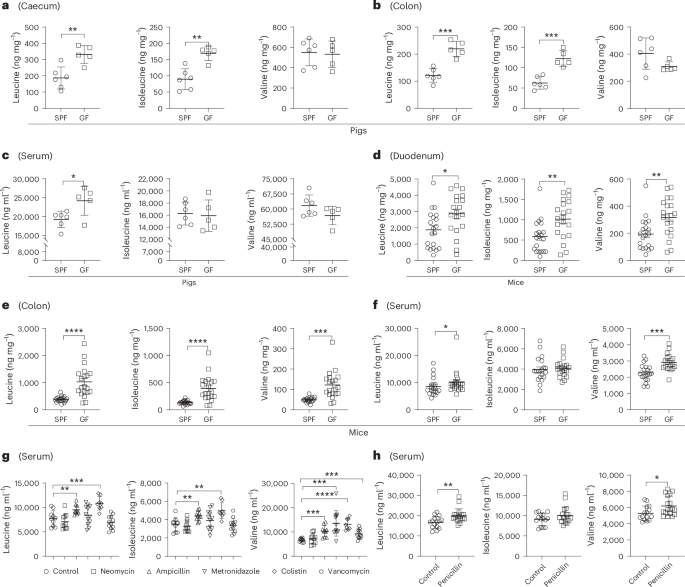
The idea for this study grew out of a recurring observation in our laboratory. Over several years, while working with both mice and pigs, we repeatedly noticed that germ-free animals exhibited markedly elevated BCAA levels compared with their conventionally raised counterparts. This increase was not limited to a single tissue or species but was consistently observed across multiple biological compartments. Initially, this seemed paradoxical. If gut microbes can synthesize BCAAs, why would their absence result in higher host BCAA levels?
This question prompted us to rethink the prevailing assumption that microbial effects on BCAA homeostasis are driven mainly by direct production or degradation in the host. Instead, we began to consider the possibility that the microbiota might influence host BCAA levels indirectly, by regulating host metabolic pathways.
To explore this idea, my group combined several complementary in vivo approaches, including antibiotic perturbation, co-housing experiments and fecal microbiota transplantation. Across all these models, altering the gut microbiota consistently reshaped circulating BCAA levels. Importantly, these effects were durable and not simply transient responses to antibiotic exposure. This reinforced our confidence that specific microbial communities actively participate in controlling host BCAA metabolism.
We then turned our attention to identifying the microbes involved. Through microbiota profiling and correlation analyses, members of the genus Lactobacillus repeatedly emerged as being negatively associated with host BCAA levels. Among them, Lactobacillus reuteri stood out. When we introduced Lactobacillus reuteri into germ-free or antibiotic-treated animals, host BCAA levels decreased significantly.
At this point, we encountered an unexpected contradiction. In vitro assays revealed that Lactobacillus reuteri produces, rather than consumes, BCAAs. This finding ruled out a simple explanation based on microbial nutrient competition and suggested that Lactobacillus reuteri must be influencing host metabolism through a more indirect mechanism. This realization became a key conceptual turning point in the project.
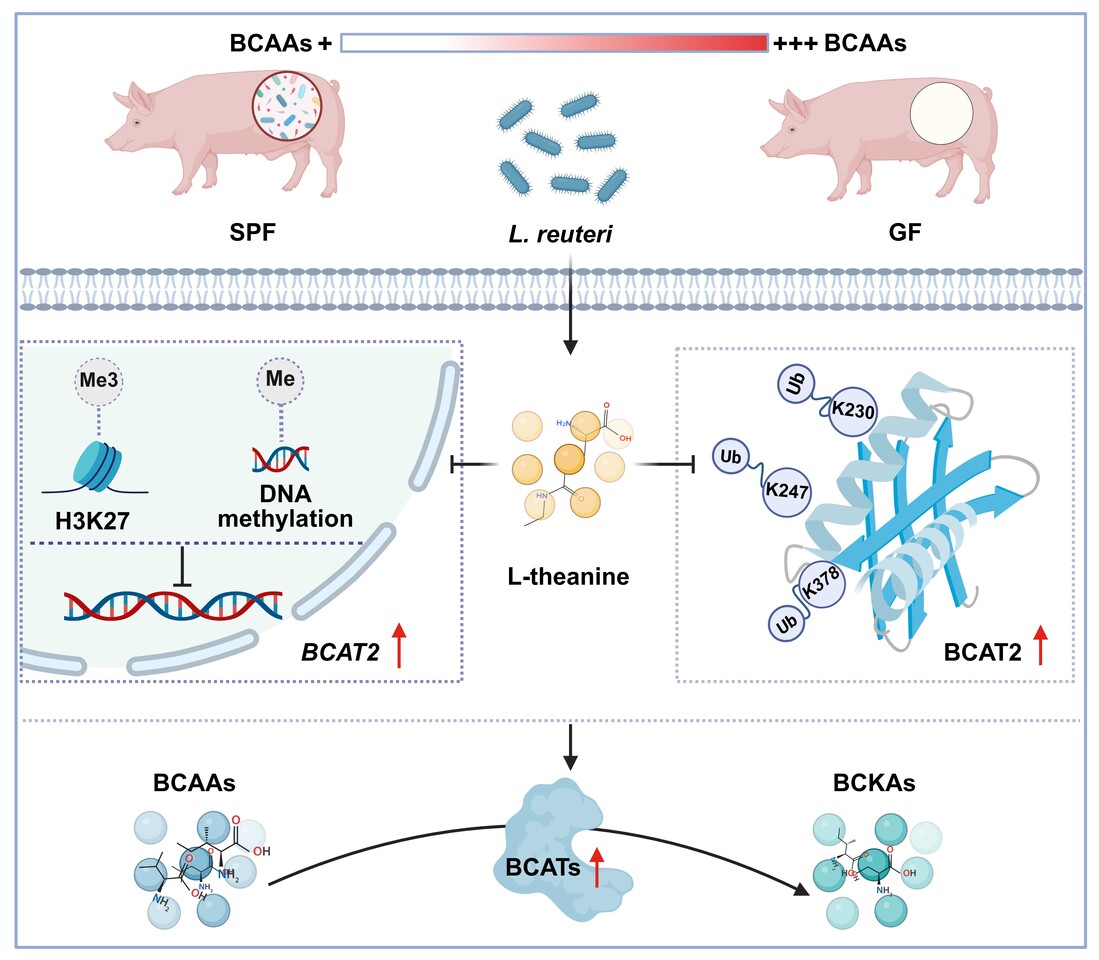
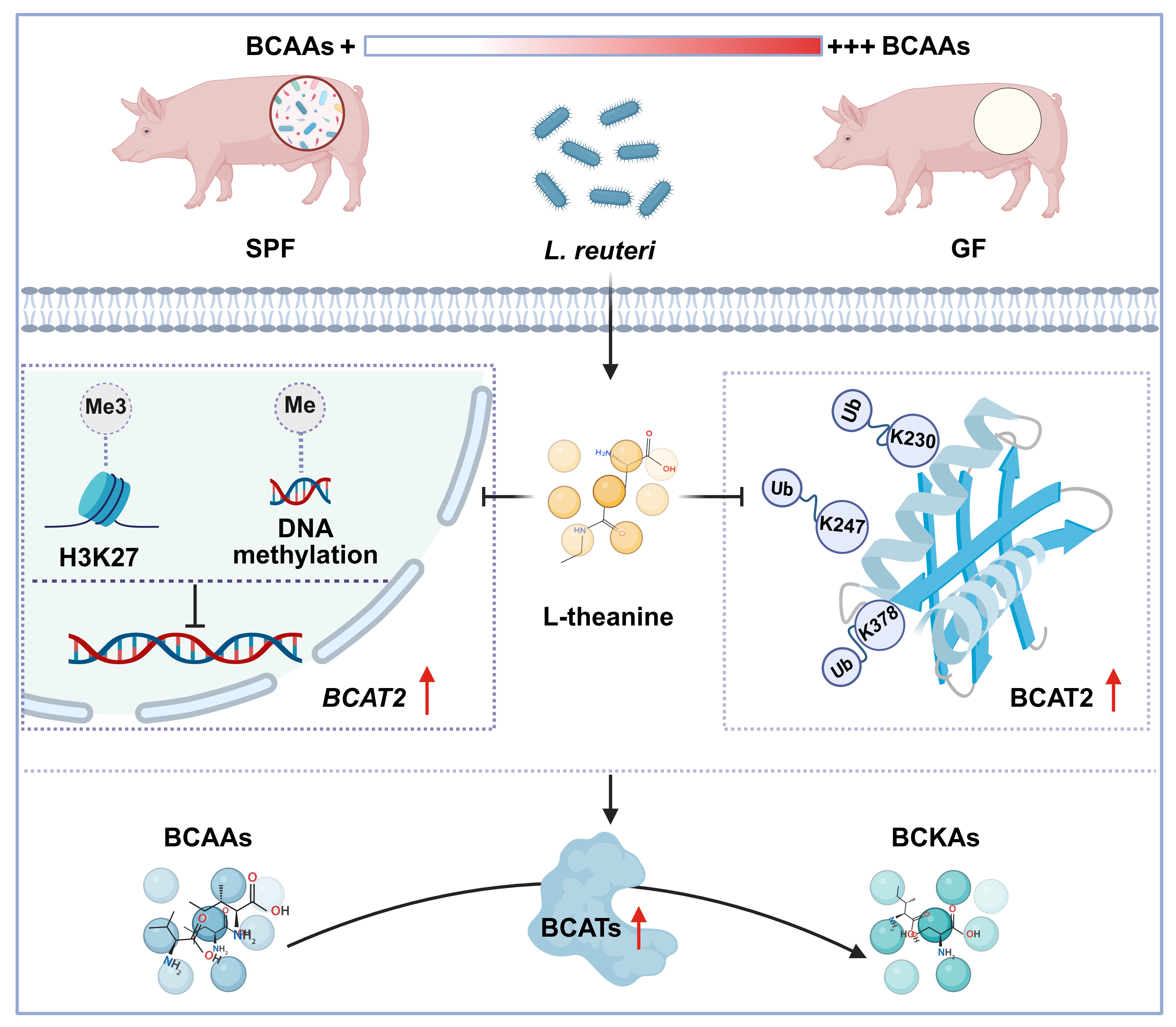
We therefore shifted our focus to microbial metabolites as potential signaling molecules. Metabolomic profiling of Lactobacillus reuteri culture supernatants highlighted L-theanine as a prominent candidate. L-theanine is best known as a bioactive compound in tea, and its role as a microbiota-derived metabolite had not been fully appreciated. We found that L-theanine levels were reduced in germ-free animals and restored upon Lactobacillus reuteri colonization.
Functional experiments confirmed that L-theanine could recapitulate the metabolic effects of Lactobacillus reuteri. In intestinal epithelial cells, L-theanine lowered intracellular BCAA levels while increasing downstream catabolic intermediates. This observation led us to focus on branched-chain aminotransferase (BCAT), the enzyme catalyzing the first and rate-limiting step of BCAA catabolism.
Mechanistic studies revealed that L-theanine regulates BCAT2 at multiple levels. It enhances BCAT2 transcription by suppressing repressive histone and DNA methylation and stabilizes BCAT2 protein by inhibiting ubiquitination at specific lysine residues. Through this coordinated regulation, L-theanine promotes host BCAA catabolism and reduces circulating BCAA levels in vivo.
A central feature of this study is that our key findings were established primarily in pigs, rather than being limited to rodent models. Pigs share closer anatomical and physiological similarities with humans, particularly with respect to intestinal architecture, nutrient absorption and metabolic regulation. By focusing on a porcine model and further validating the underlying mechanisms in porcine small intestinal epithelial cells, our work provides a physiologically relevant framework for understanding microbiota-driven regulation of BCAA metabolism and offers insights that may be directly informative for human metabolic health and disease.
More broadly, our findings point to a previously unrecognized microbiota–host axis regulating BCAA metabolism. Given the strong association between elevated BCAA levels and metabolic disease, microbial metabolites such as L-theanine may represent attractive targets for metabolic intervention. As L-theanine is highly enriched in tea, one of the most widely consumed beverages worldwide, our work suggests a potential link between gut microbiota, dietary tea intake and metabolic homeostasis.
As one reviewer succinctly commented, “This is an interesting study (and a tour de force) that provides novel mechanistic insights into how gut bacteria may influence BCAA in the host.” We hope that this study will encourage further exploration of microbial metabolites as regulators of host amino acid metabolism and inspire new perspectives on the metabolic functions of the gut microbiota.
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in