CD2: A Plug-and-Play CAR-T Upgrade Strategy to Overcome Exhaustion and Enhance Anti-Tumor Potential
Published in Biomedical Research
Why This Research Matters
T cells are like well-trained soldiers in our body's defense force, helping us fight off invaders like infections and cancer. CAR-T cell therapy gives these soldiers special gear – called a chimeric antigen receptor (CAR) – that helps them target harmful cells much more precisely. This approach has been a game-changer for treating blood cancers like leukemia, lymphoma, and multiple myeloma.
But here's the problem: These upgraded soldiers often struggle to hold their ground against the enemy during long fights. They eventually get worn out ('exhausted'), and that's a big reason why so many patients relapse after CAR-T therapy. Our research tackles this challenge head-on: Can we give them some extra help to overcome these hurdles? This is where CD2 – a natural tool T cells already have in their kit – comes into play.
The Spark That Started It All
We have been exploring strategies to enhance the resilience and efficacy of CAR-T cells against tumors. The formation of immunological synapse (IS) is a prerequisite for T cell-mediated anti-tumor responses – analogous to soldiers engaging enemies at close range, where IS serves as the cellular "grip". Critically, CAR-mediated IS exhibits reduced structural integrity compared to that formed by the native T cell receptor (TCR). Within the TCR system, the CD58-CD2 interaction is fundamental for establishing high-quality IS. Our previous work demonstrated that CD58 deficiency on tumor cells impairs IS integrity in CAR-T systems. This finding prompted us to hypothesize that suboptimal CD2 expression on T cells may limit their ability to form robust IS. Could augmenting CAR-T cells with CD2 – effectively strengthening the soldiers' grasp – enhance their anti-tumor potency? This question became the seed of our project, driven by scientific inquiry and the urgent need to help patients refractory to current therapies.
The Sweet Taste of Success
Our multidimensional analyses revealed CD2's critical role in T-cell biology: its expression is low in resting T cells but surges upon activation, with higher levels in effector versus naïve/central memory subsets and greater abundance in CD8⁺ compared to CD4⁺ T cells. Most critically, under repeated tumor stimulation, CAR-T cells exhibited progressive CD2 depletion as exhaustion intensified. Database analyses further suggested CD2's important role: Patients with higher CD2 expression displayed stronger immune activation signatures and enhanced T cell recruitment potential. Across multiple cancer types, CD2 levels strongly correlated with tumor-infiltrating lymphocyte (TIL) abundance. Higher CD2 expression was associated with longer overall survival and reduced mortality risk. In our DLBCL CAR-T cohort, treatment-resistant patients trended towards lower CD2 expression.
Mechanistically, CD2 operates through dual pathways: It remodels the immunological synapse (IS) by boosting F-actin polarization, enhancing synaptic stability to optimize tumor engagement. Following antigen stimulation, CD2-CAR-T cells downregulate exhaustion-associated factors like NR4A1/2/3 compared to conventional CAR-T cells.
Functionally, CD2-CAR-T cells demonstrated marked therapeutic superiority: In repeated stimulation models, they exhibited stronger resistance to exhaustion compared to conventional CAR-T cells. They maintained high killing efficacy against tumors with low antigen density. Competitive in vivo experiments confirmed significantly enhanced proliferative capacity.
Collectively, these advantages open new avenues for novel optimization strategies to enhance CAR-T efficacy.
What’s Next?
What does this mean for the future? Our research shows that adding the CD2 component could be a game-changer for CAR-T cell therapy, giving these cancer-fighting cells a major boost. Imagine it like equipping soldiers with tougher armor and longer-lasting energy packs – enabling them keep up the fight against cancer for much longer.
The best part? No need to start from scratch. Instead, it’s a precision upgrade that strengthens the weakest parts in existing treatments. This makes it much easier and faster to move this new approach from the lab to real patients.
We are now advancing CD2-enhanced CAR-T therapy into clinical trials. These exciting results are just the beginning. We hope this sparks a wave of new ideas and collaborations among scientists worldwide.
Wrapping Up
This research journey has been a true rollercoaster. We faced moments of self-doubt and near defeat—especially during those painstaking months when repeated experiments consistently failed to show that CD2 overexpression could significantly enhance CAR-T cells' short-term tumor-killing ability.
Then came a serendipitous discovery: While investigating how tumor cells lose CD19 markers (a common immune escape mechanism), we checked Raji cells after co-culture with different CAR-T cells. To our surprise, Raji cells better maintained their CD19 surface markers when exposed to our CD2-CAR-T-19 cells—unlike the rapid CD19 loss seen with conventional CAR-T cells.
This revelation redefined our understanding:
The preserved CD19 markers indicated that CD2 fundamentally alters the cell-cell interface between CAR-T and tumors. This prompted critical questions about the underlying mechanism:
Does CD2...
① Strengthen synaptic engagement?
→ Potentially improving target cell adhesion
② Reduce abortive interactions?
→ Minimizing energy waste from failed killing attempts
③ Heighten antigen sensitivity?
→ Overcoming low-antigen tumor evasion
We put these ideas to the test, and these mechanistic insights culminated in our completed study. I take profound pride in this work and extend deepest gratitude to my colleagues.
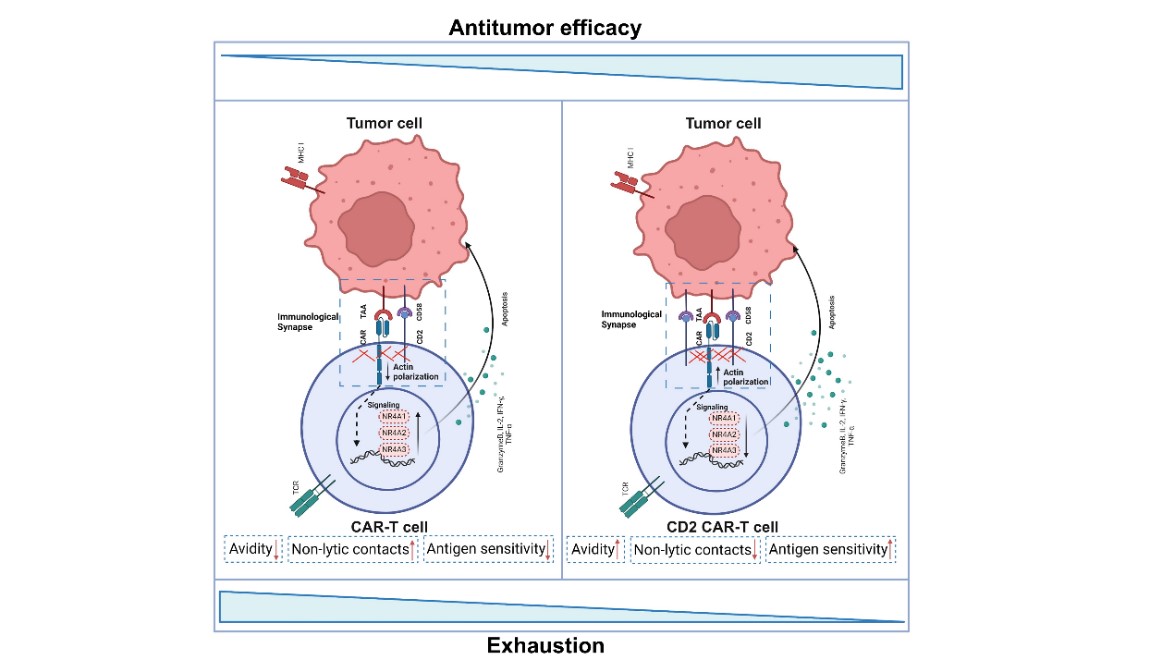
Schematic Diagram.
Ectopic CD2 expression in CAR-T cells sustains elevated CD2 levels, significantly enhancing immunological synapse quality, improving long-term antitumor efficacy, and mitigating T cell exhaustion. CD2 stabilizes T cell–tumor cell interactions and promotes sustained synaptic F-actin remodeling. CD2 overexpression reduces exhaustion by downregulating NR4A proteins. CD2 co-expression lowers critical antigen density thresholds.
Follow the Topic
-
Cancer Cell International

This journal publishes articles on all aspects of cancer cell biology originating from work using laboratory experimentation.
Related Collections
With Collections, you can get published faster and increase your visibility.
Cell competition in tumorigenesis
Survival of the fittest! Sadly this also applies to cancer cells. In the early phase of cancer, (epi)genetic mutations in initiated cells may be selected for their ability to exploit cell competition to eliminate neighboring less competent cells, thereby facilitating tumor expansion.
These tumor cells' competitive skills include but are not limited to the ability to live under hypoxia and acidic microenvironment, clonal expansion of cancer stem cells that contributes to tumor heterogeneity, as well as evading host immune cell surveillance. Interestingly, it has been recently shown that cell competition can also occur in later phases of cancer.
More importantly, cancer risk factors (such as unhealthy diet and chronic inflammation) could also produce variations of niche supplying the tumor and might dictate the outcome of cell competition in tumorigenesis.
It is anticipated that strategies pinpointing competitive cell interactions in various phases and microenvironments of cancers might help to suppress cancer initiation, expansion, and progression. In this collection, we welcome original articles focusing on tumor cell competition research.
Publishing Model: Open Access
Deadline: Ongoing
Onco-proteogenomics
With the advancement of sequencing technologies, whole genome sequence information can be obtained robustly in a fairly short time and at low cost. Yet, currently there is often a lack of connection of genomic data to proteomic data, probably due to the fact that standard proteomics workflows rely on databases of only known wild-type/canonical proteins, but these databases generally do not integrate the data of cancer-associated proteins with countless amino acid sequence alterations due to gene mutations, post-translational modifications, and aberrant chromosome rearrangement. Therefore, in the next phase, it becomes extremely important to integrate experimentally-generated Mass Spectrometric data accordingly with the genomic information to identify tumor-specific proteins and isoforms.
Onco-proteogenomics has great promise and holds the key in improving our understanding of cancer biology and it is anticipated that the routine utilization of this integrated omics approach would become one of the gold standards of precision oncology. In this collection, we welcome original articles focusing on cancer proteogenomics research.
Publishing Model: Open Access
Deadline: Ongoing



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in