Cell bioengineering for neurogenetics in developmental disorders.
Published in Bioengineering & Biotechnology, Neuroscience, and Cell & Molecular Biology

This month while following-up on my research interests, I read about a study seeking for bioengineers to carryout cell bioengineering for neurogenetics, which inspired me to connect a few research-related dots on this write-up. As detailed here and here, miniature microfluidic organ-chip instruments reiterate a variety of disease genotypes in microphysiological environments to recreate pathological pathways in vitro. While exploring diverse chip types designed for heart disease, kidney, lung, duodenum, and the brain, I noticed of their potential to explore the underlying neurogenetics of inherited neurodevelopmental disorders. Of note, researchers have already developed a wealth of robust animal models to investigate mutational mechanisms in severe neurological disorders and gather insights to clinical enigmas [Zoghbi 2011].
In this post, I focus on a few core animal models that have successfully mimicked clinical paradoxes, to understand how such studies can guide in vitro cell bioengineering with neural organoids, to emulate developmental disorders on a microphysiological platform with patient-derived tissues.
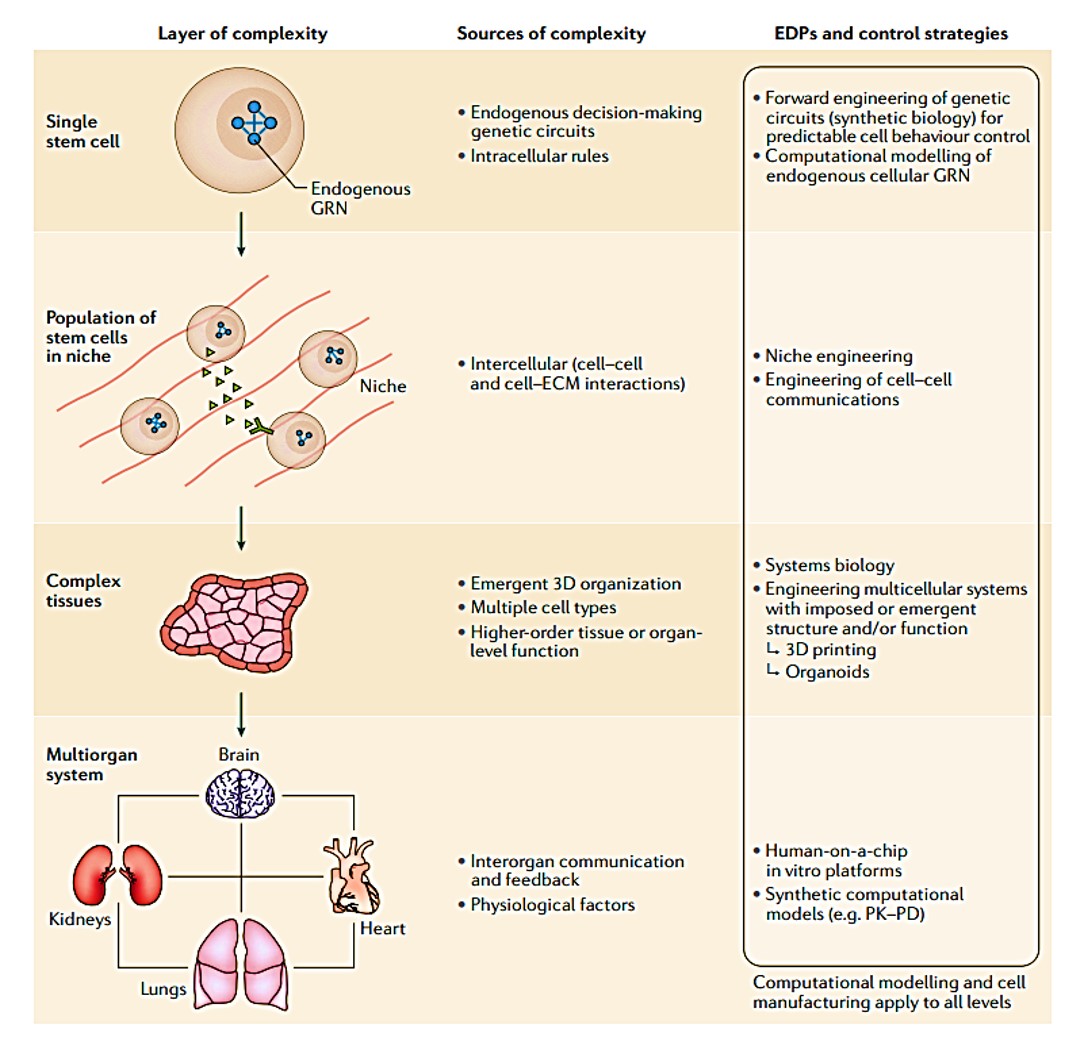
Figure 1: layers of complexity in stem cell inclusive cell bioengineering systems. Credit: [Tewary 2018].
From animal models to neural organoids
Mice, Drosophila, zebrafish, C. elegans, and yeast experimental models are useful to test the mechanistic hypothesis of neuroscience, for eventual evaluation in human subjects. Studies have already shown the possibility of reversing Rett syndrome-like phenotypes in a mouse model of the disease, by activating a silenced Mecp2 gene; fueling the notion (and the hype) that incurable developmental disorders such as Rett syndrome or fragile X syndrome may be therapeutically approachable [Guy 2007].
Most early insights gained from animal models can be validated with human tissue to develop clinical diagnostics, thus bringing to light the capacity to explore neurogenetics via cell bioengineering efforts in vitro [Samarasinghe 2021]. While mouse behavioral models of neurological disorders are very useful for preclinical drug testing, systemic tests are not ideal in mouse models due to their size and docile nature, which prevents the assessment of specific measurable characteristics of behavior, this is of course a common limitation with in vitro assessments as well.
Nevertheless, cell bioengineering efforts have successfully explored neurogenetics with patient-derived human tissues such as neural organoids, and via stem cell bioengineering efforts [Park 2024, Tewary 2018] to gain valuable insights to gene silencing methods, to understand disease pathways and to explore the subsequent therapeutic approachability and reversibility of some X-inherited neurodevelopmental disorders [Basta 2023, Guy 2007].
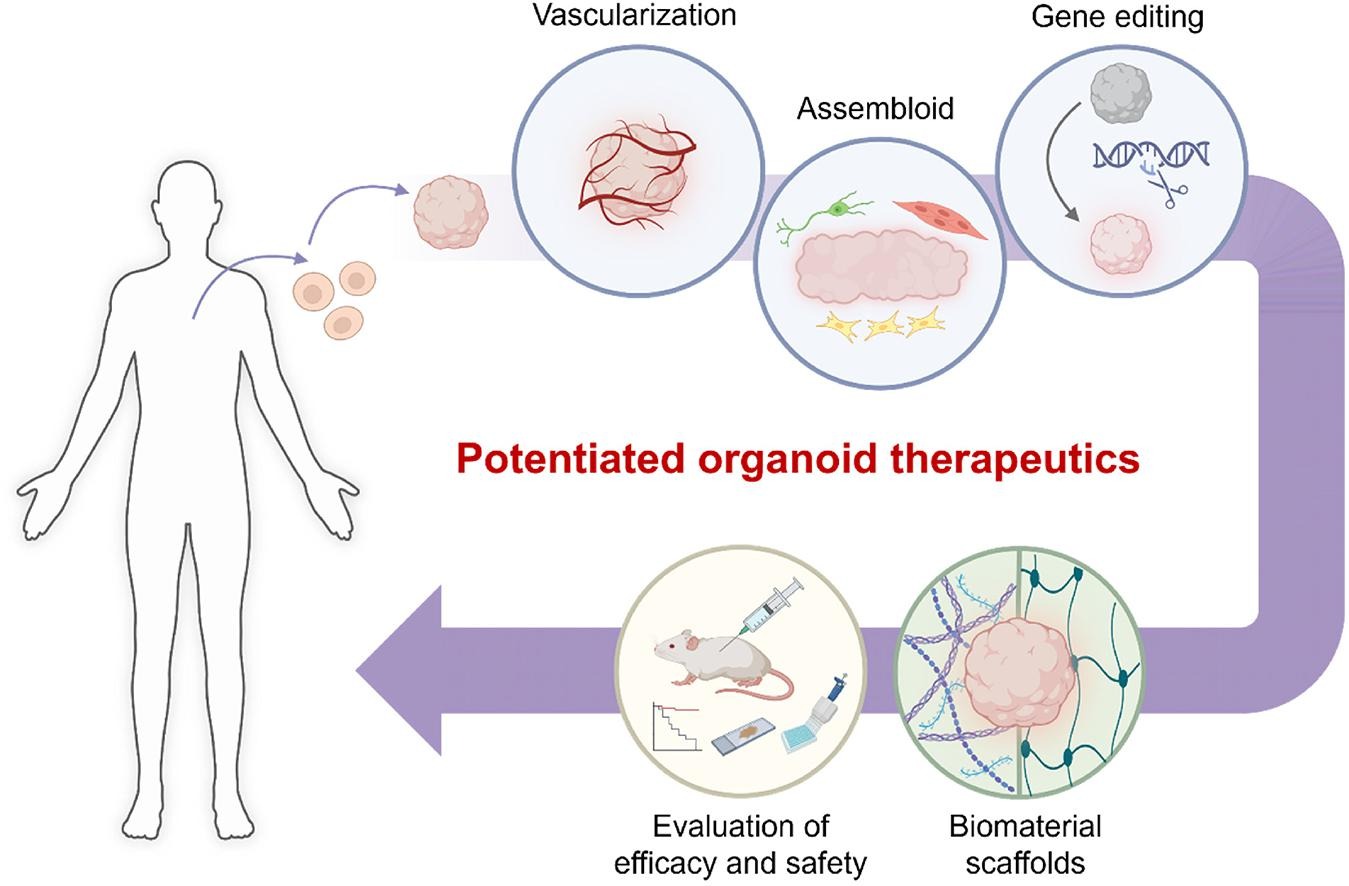
Figure 2: Bioengineering toolkits to potentiate organoid therapeutics. Credit: [Park 2024].
The key to disease-oriented research
Study outcomes have highlighted that the key to successful disease-oriented research is based on methodical and in-depth functional analyses of culprit proteins by using disease models to investigate physiological and pathological consequences of dysfunctional proteins [Zoghbi 2011]. Neuroscientists have already made headway in disease-oriented research by investigating the reversibility of developmental and degenerative/psychiatric disorders in mouse and Drosophila models [Fortini 2009].
This trajectory is highlighted by analyzing protein-protein interaction networks underlying neurological disorders such as ataxia (Figure 3) [Lim 2006]. The advent of similar protein structure prediction models such as AlphaFold aim at structure-based screening of protein-protein interactions to identify a pair of disease-related candidate proteins more accurately [Mischley 2024]. The recent focus on protein engineering by developing mRNA with chemically modified poly A tails to yield higher mRNA levels in cells, is a similar translational treatment strategy to edit genes or replace faulty proteins in the long-term [Chen 2024].
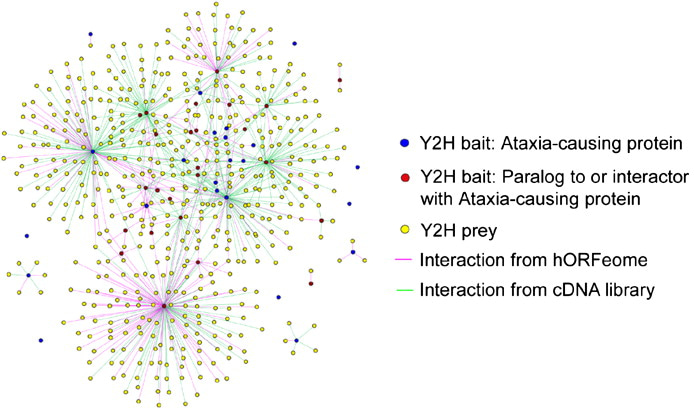
Figure 3: Generating a proteome-scale interactome network for inherited ataxia with gateway-compatible yeast two-hybrid (Y2H) destination vectors, to generate fusion proteins of ataxia constructs to analyze the neurological disorder. Credit: [Lim 2006].
Despite gross anatomical differences between humans and flies, researchers have succeeded in using Drosophila species to efficiently replicate a variety of pathogenic neurological disorders [Greene 2003]. Studies exploring the loss of parkin function associated with Parkinson disease with Drosophila parkin mutants, for instance, have revealed the significance of parkin for mitochondrial function [Greene 2003, Clark 2006]. Such outcomes can be recapitulated with neurological architecture using patient-derived cell types via cell bioengineering to similarly explore the underlying neurogenetics.
The basis of the experiments
While many neurodegenerative diseases are linked to causative proteins, when does a protein contribute to disease via gain-of-function? And when is it accrued via loss-of-function? Although the pathological loss-of-function mechanism is generally straightforward to recreate in the lab, the etiology behind gain-of-function disorders is more obscure [Kratter 2010]. Since the latter can result in a novel property emerging in a disease-associated protein or enhance the regular function of a protein to toxic levels. The nature of gain-of-function mutations can be explored relative to monogenic diseases that can be validated with disease models [Orr 2007].
Existing work has already explored in vivo models of polyglutamine (polyQ) diseases that span at least nine neurodegenerative disorders caused by the pathological expansion of CAG trinucleotide repeats in the coding region of different genes [Orr 2007]. This genotype represents the most common cause of inherited neurodegenerative disorders.
The polyQ disease of spinobulbar muscular atrophy is an X-linked neurodegenerative disorder, where polyQ expansion occurs in the androgen receptor; a classic nuclear hormone receptor, thus making the function of the mutant proteins well understood [Kratter 2010]. Studies have shown the capacity to combine existing knowledge of the androgen receptor with the power of fly genetics (Drosophila) to expand the understanding of polyQ toxicity [Nedelsky 2010].
Neural organoids from neuroscience to cell bioengineering
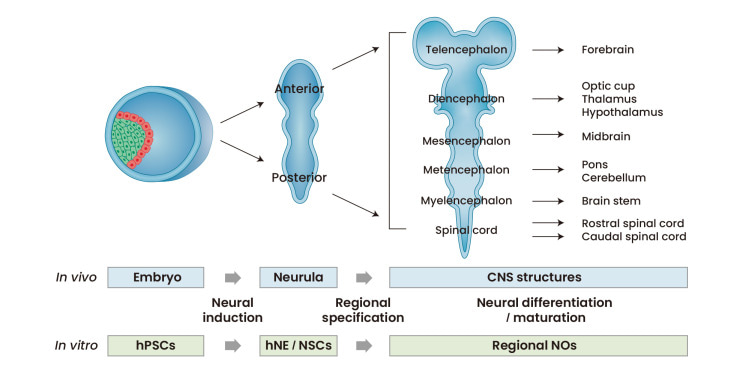
Figure 4: The strategy of generating neural organoids in the lab is based on the process of neural development in vivo. Credit: [Lee 2022]
Neural organoids offer a versatile platform with 3-D cultures of human neural tissue or organ-like structures by mimicking in vivo developmental processes in vitro [Lee 2022] (Figure 4). The tissues exhibit aspects of brain histoarchitecture, resulting in an explosion of stem cell inclusive culture methods to facilitate new discoveries [Warmflash 2014, Qian 2016]. Neuroscientists and bioengineers have developed cerebral organoids as 3-D cultures to model organogenesis and investigate human brain development [Qian 2016].
Existing protocols include forebrain specific organoids generated from human induced pluripotent stem cells, and the recapitulation of the midbrain and hypothalamic organoids to encompass neurogenesis, alongside gene expression, to induce disease pathways and explore neurodevelopmental and neurodegenerative diseases, such as infection-based microcephaly via Zika virus exposure [Qian 2016].
The stages of neural organoid generation contain 3-4 steps that mimic the first principles of in vivo developmental cascades. Bioengineers typically induce human pluripotent stem cells into neuroepithelia-like cell populations by applying culture factors/components to determine neural fates. The process can result in the induction of anterior and posterior neural parts via diverse developmental processes, followed by further specification of 3-D neural clusters into specific domains [Lee 2022]. These neural organoids can be cultured under appropriate conditions for neural differentiation, advanced neural development, and long-term maturation steps, as suited for cell bioengineering experiments.
Brain organoids – the methods of inquiry
Complex neural organoid models can be formed via four key methods: fusion, assembly, connection, and polarization (Figure 5) [Lee 2022]. Neural organoids have successfully replicated phenotypes of human genetic mutations of microcephaly that are not faithfully replicated in mouse models [Lancaster 2013]. Table 1 (zoom in) highlights studies on the successful recapitulation of neurological diseases using neural organoids.
Since whole genome screening sheds light on rare genetic mutations that underly developmental brain disorders, the -omics related data can inform gene editing efforts and organoid development protocols to recreate rare genetic disorders in the lab [Lee 2022]. Bioengineered neuronal organoids can recapitulate neuronal plasticity to resemble giant depolarizing potential-like events in early neural networks and mimic the neural activity of the fetal brain [Zafeiriou 2020].
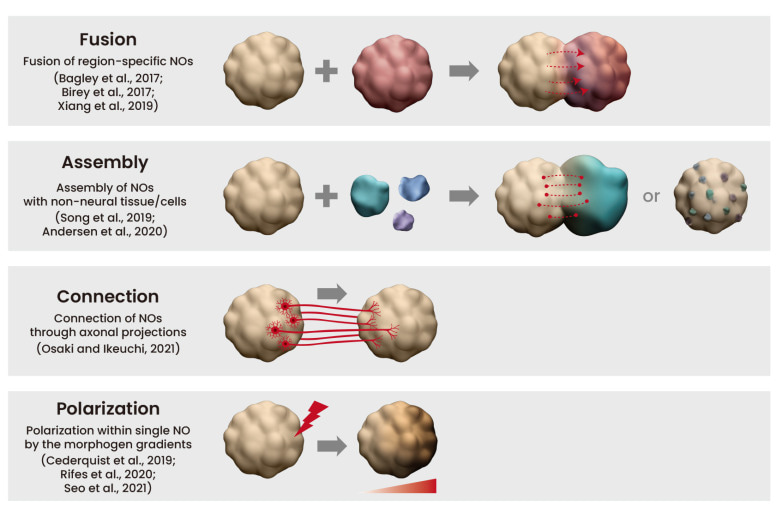
Figure 5: Complex neural organoid (NO) models. Credit: [Lee 2022].
In alignment with successful, pioneering animal studies, bioengineers have recently used induced pluripotent stem cells derived from individuals with Rett syndrome to show highly abnormal, epileptiform changes and activity, alongside transcriptomic differences at the single-cell level [Guy 2007, Samarasinghe 2021].
While epileptic episodes can be recognized on a culture dish due to altered neuronal burst activity and local field potentials, to successfully model Rett syndrome and schizophrenia from patient-derived induced pluripotent stem-cell based neural organoids [Lee 2022]. The use of organoids to model psychiatric disorders is more challenging due to the associated abnormalities of sophisticated pathologies, which include neural circuit formation and synaptic plasticity without gross signs of structural changes [Spronsen 2010]. Despite their significance as powerful bioengineering tools, questions remain as to if neural organoids, which provide a wealth of advantages, also possess sufficient complexity to model disease conditions.
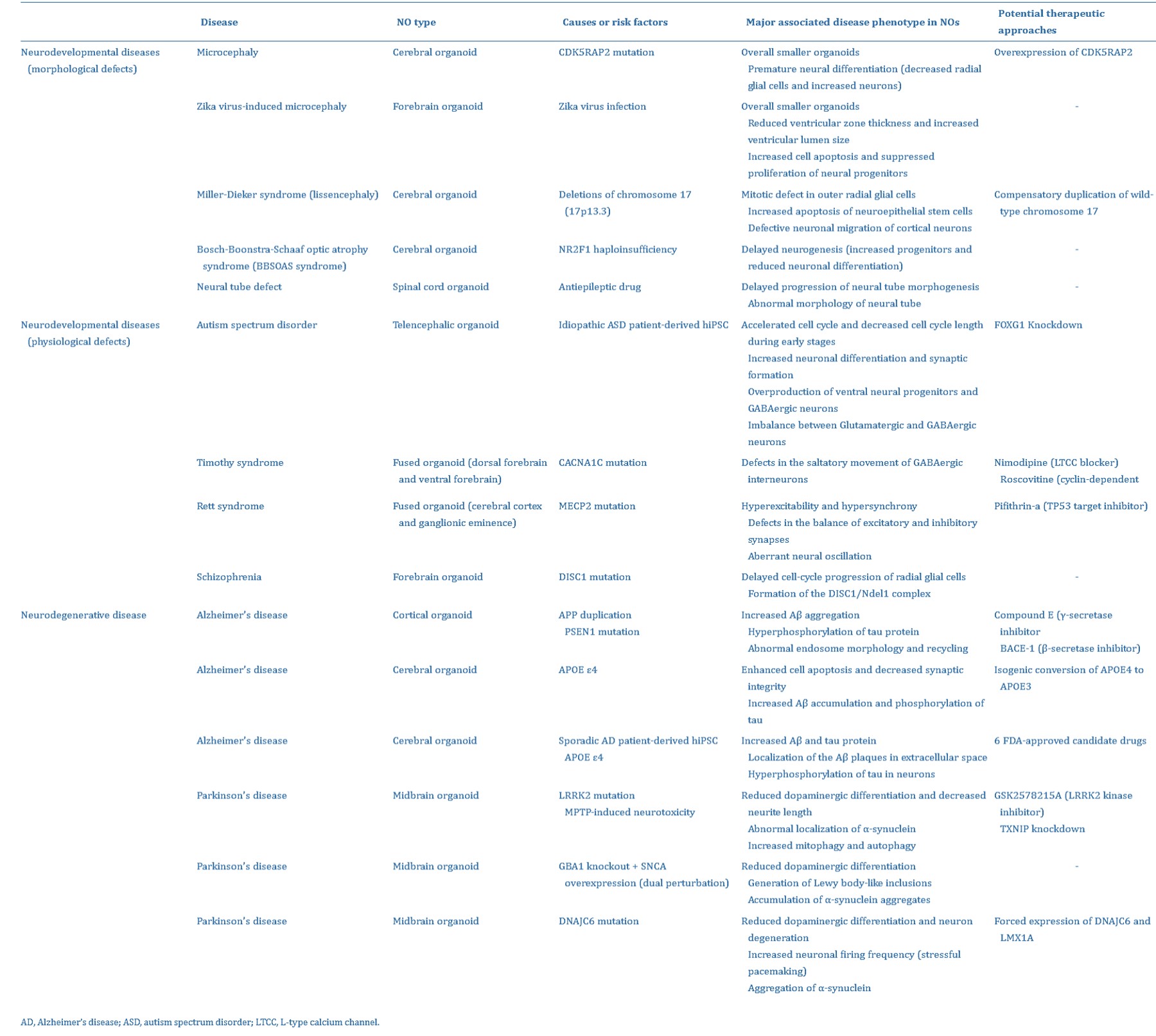
Table 1: The successful recapitulation of neurological diseases using neural organoids [Lee 2022].
To wrap things up: combining microfluidics and neural organoids
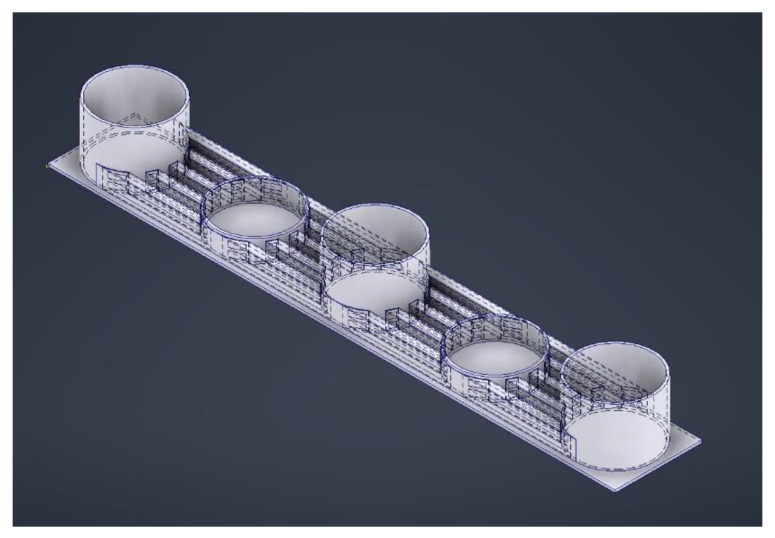
Figure 6: Designing a microfluidic device with five wells. The deeper wells serve as media reservoirs and the shallower regions house the organoids, with interconnecting channels to exchange nutrients and waste, while limiting shear stress for optimized brain organoid growth. Credit: [Hetzel 2023].
Bioengineers are skilled at analyzing problems and devising solutions; therefore, the question of neural organoid viability can be assessed by combining microfluidics and organoids: somewhat aptly referred to as the power couple of developmental biology, due to their capacity to unlock deeper understandings of in vivo cellular processes [Hetzel 2023]. Microfluidic instruments that are used for organ-on-chip instruments can support patient-derived organoids and spheroids in two ways [Dadgar 2020]:
- The microfluidic device can be designed for use with single cells to induce the formation of organoids, or
- The device can be designed to inject fully formed organoids into the device for filtering and manipulation experiments.
The intricacy of the nervous system and its complex fluid interactions can be emulated relative to both blood flow and cerebral spinal fluid, to facilitate bidirectional flow [Cho 2021]. Microfluidic flow forms a critical step to develop several features of neurons and recreate attributes of the nervous system with organoids (Figure 6) [Hetzel 2023]. To fully appreciate their versatility in-depth, however, requires a separate stand-alone article.
Simply put, since brain organoid development occurs across various platforms at different stages of development, the process can be physically harmful to the tissue (Figure 4, above). To prevent such issues, a microfluidic device can be tailored with microwells to isolate organoids from each other and force an open-air interface with an integrated mesh membrane in the well, to prevent a hypoxic or necrotic core, and ensure effective neurogenetics analyses outside the setup [Hetzel 2023] (Figure 7). This feature can elevate organoids for media changes, without damaging the tissue during neural differentiation or growth, eliminating the need to transfer to different platforms [Ao 2020].
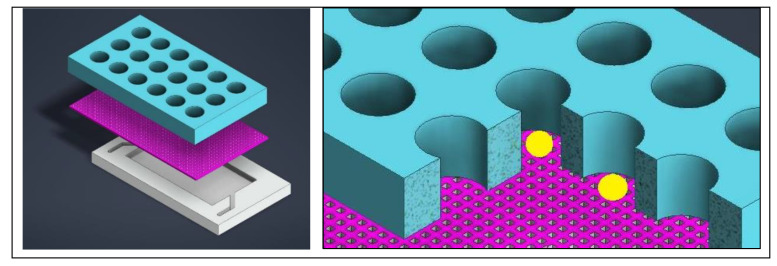
Figure 7: Designing a mesh-integrated microfluidic-organoid composite device for optimized development of organoid (seen in yellow) histoarchitecture. Credit: [Hetzel 2023].
To wrap things up, another key criterion for organoid development is vascularization, where the design of a microfluidic device with flow on two sides can induce a vascular network with interstitial flow and stresses comparable to human capillaries [Hetzel 2023]. The design and development of the human nervous system, and accompanying drug screening applications on a chip define two very different uses of organoids, and therefore warranty two very different microfluidic device designs. In conclusion, table 2 outlines a general summary of microfluidic-organoid composite possibilities, applicable to emulate neurological developmental disorders as accurately as possible, for subsequent neurogenetics investigations.
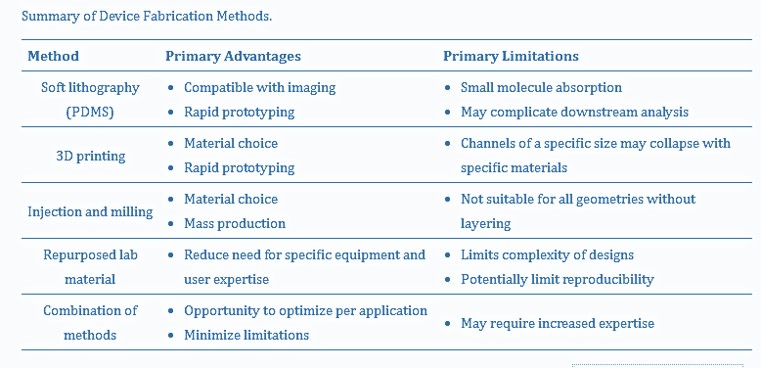
Table 2: A summary of organoid-encapsulated device fabrication methods [Hetzel 2023].
Header Image: Brain organoids replicate key events in human brain development. Neuroscience, Broad Institute.
References:
- Zoghbi H. et al. Neurogenetics: Advancing the “Next-Generation” of Brain Research, Neuron, 2010.
- Tewary M. et al. Stem cell bioengineering: building from stem cell biology, Nature Reviews Genetics, 2018.
- Guy J. et al. Reversal of neurological defects in a mouse model of Rett syndrome, Science, 2007.
- Samarasinghe R. et al. Identification of neural oscillations and epileptiform changes in human brain organoids, Nature Neuroscience, 2021.
- Park S. et al. Bioengineering toolkits for potentiating organoid therapeutics, Advanced Drug Delivery Reviews, 2024.
- Basta M. et al. Genetics, X-Linked Inheritance, StatPearls Publishing, 2024
- Fortini M. Notch signaling: the core pathway and its posttranslational regulation, Developmental Cell, 2009.
- Lim J. et al. A protein-protein interaction network for human inherited ataxias and disorders of Purkinje cell degeneration, Cell, 2006.
- Mischley V. et al. PPIscreenML: Structure-based screening for protein-protein interactions using AlphaFold, BioRxiv, 2024.
- Chen H. et al. Branched chemically modified poly(A) tails enhance the translation capacity of mRNA, Nature Biotechnology, 2024.
- Green J. et al. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants, PNAS 2003.
- Clark I. et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin, Nature, 2006.
- Kratter, I. et al. PolyQ Disease: Too Many Qs, Too Much Function? Neuron, 2010.
- Orr H. et al. Trinucleotide repeat disorders, Annual Review Neuroscience, 2007.
- Nedelsky N. et al. Native functions of the androgen receptor are essential to pathogenesis in a Drosophila model of spinobulbar muscular atrophy, Neuron, 2010.
- Lee J. et al. Neural Organoids, a Versatile Model for Neuroscience, Molecules and Cells, 2022
- Warmflash A. et al. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells, Nature Methods, 2014.
- Qian X. et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure, Cell, 2016.
- Lancaster M. et al. Cerebral organoids model human brain development and microcephaly, Nature, 2013.
- Zafeiriou M. et al. Developmental GABA polarity switch and neuronal plasticity in Bioengineered Neuronal Organoids, Nature Communications, 2020.
- Spronsen M. et al. Synapse pathology in psychiatric and neurologic disease, Current Neurology and Neuroscience Reports, 2010.
- Hetzel L. et al. Microfluidics and Organoids, the Power Couple of Developmental Biology and Oncology Studies, MDPI, 2023.
- Dadgar N. et al. A microfluidic platform for cultivating ovarian cancer spheroids and testing their responses to chemotherapies, Microsystems and Nanoengineering, 2020.
- Cho A. et al. Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids, Nature Communications, 2021.
- Ao Z. et al. One-Stop Microfluidic Assembly of Human Brain Organoids To Model Prenatal Cannabis Exposure, Analytical Chemistry, 2020.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in