Combination therapy targeting Erk1/2 and CDK4/6i in relapsed refractory multiple myeloma
Published in Cancer
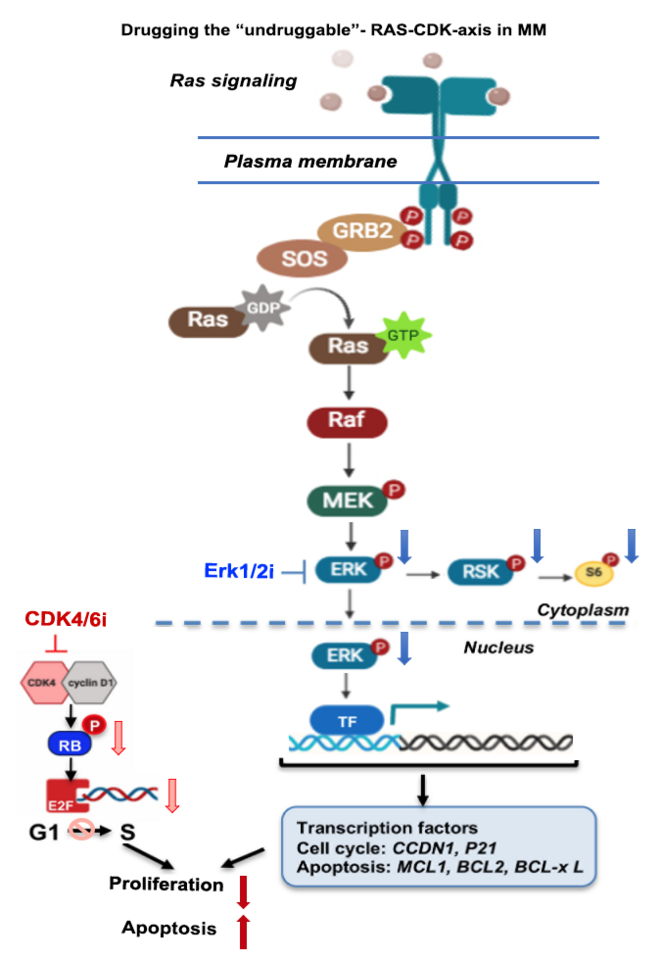
Multiple Myeloma (MM) remains incurable despite recent therapeutic advances, which have dramatically improved patient survival within the last decade, increasing life expectancy from 3 to 10 years [1]. However, novel therapies are urgently needed due to the high frequency of relapse and the development of drug resistance. Targeting oncogenic RAS-RAF-MEK-ERK and CDK pathway therapeutically in MM has long been a high priority because RAS activating mutations and alterations in Cyclin-D pathways remain as one of the most frequent recurrent genetic lesions in MM patients [2-5]. These alterations are present at the time of diagnosis in ~25% of patients, and this percentage dramatically increases to 75% of patients at the time of relapse. In our recently published manuscript in Leukemia, we examined treatment effects of novel Erk1/2 and CDK4/6 inhibitor combination treatment (Erk1/2i+CDK4/6i) on MM cell killing [6].
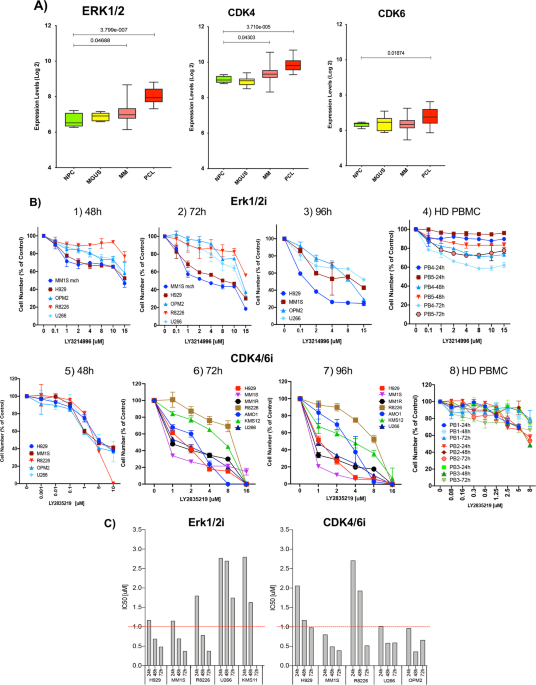
As part of this study, we found that treatment of MM cells with the Erk1/2 and CDK4/6 inhibitors, in a dose- and time-dependent manner, arrested MM-cells in G0/G1 phase, hindered uncontrolled cell proliferation, and induced mitochondrial-dependent MM cell apoptosis. The synergistic effects of this treatment combination attained selective clonal MM cell killing with enhanced cytotoxicity and minimal side effects. These effects were maintained even when patient-derived MM cells were treated in the context of autologous bone marrow stromal cells, which can trigger MM cell growth and drug resistance. Our study also shows that cells from healthy donors were relatively resistant to Erk1/2i+CDK4/6i treatment, suggesting that this combination therapy could offer a favorable therapeutic profile[6].
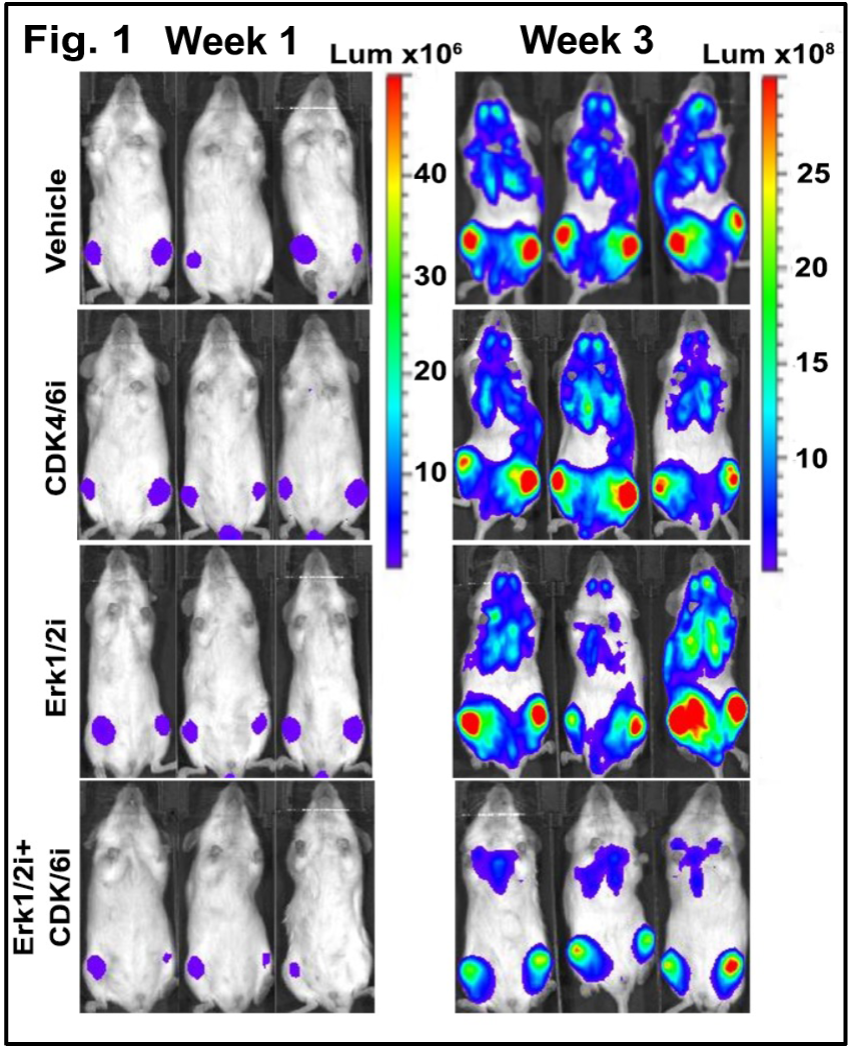
We then tested this combination Erk1/2i+CDK4/6i treatment in vivo in a disseminated MM model. This experiment demonstrated that Erk1/2i+CDK4/6i combination therapy supported the promising results from the in vitro studies. Tumor burden was significantly lower in mice treated with the combination therapy when compared with either drug alone or the control (tumor progression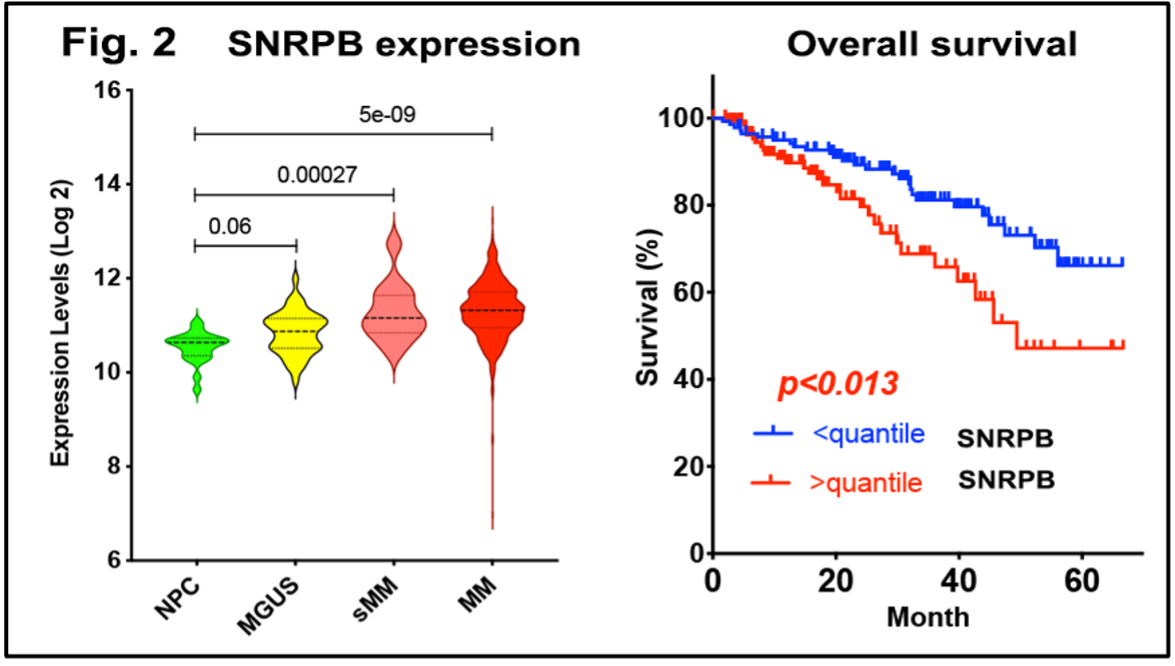 monitored by bioluminescence imaging; Fig 1). Transcriptome analyses of BM cells from treated mice suggested that the combination of these inhibitors can overcome BM-cell mediated resistance in vivo. Additionally, we observed no significant change in the bodyweight of mice treated with this combination therapy, echoing the clinical and therapeutic viability demonstrated in the in vitro studies[6].
monitored by bioluminescence imaging; Fig 1). Transcriptome analyses of BM cells from treated mice suggested that the combination of these inhibitors can overcome BM-cell mediated resistance in vivo. Additionally, we observed no significant change in the bodyweight of mice treated with this combination therapy, echoing the clinical and therapeutic viability demonstrated in the in vitro studies[6].
Most significantly, our study identified Erk1/2i+CDK4/6i treatment-associated genes, including SNRPB. Overexpression of SNRPB is associated with shorter overall survival (OS) of MM patients. This gene encodes one of the subunits of the U1 spliceosome complex and is involved in altered RNA splicing, specifically, in the intron retention process [7, 8]. This process is a unique marker of malignant transformation.
In summary, our studies 1. showed the synergistic anti-MM effect of combination Erk1/2i+CDK4/6i both in vitro and in vivo and 2. identified biomarkers associated with response to this treatment. Taken together, these data provide the preclinical framework for the evaluation of Erk1/2i+CDK4/6i combination therapy for the targeting of Ras and CDK in order to improve patient outcomes in MM.
- Kumar, S.K., et al., Multiple myeloma. Nat Rev Dis Primers, 2017. 3: p. 17046.
- Lohr, J.G., et al., Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy. Cancer Cell, 2014. 25(1): p. 91-101.
- Bolli, N., et al., Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat Commun, 2014. 5: p. 2997.
- Urashima, M., et al., Role of CDK4 and p16INK4A in interleukin-6-mediated growth of multiple myeloma. Leukemia, 1997. 11(11): p. 1957-63.
- Canavese, M., L. Santo, and N. Raje, Cyclin dependent kinases in cancer: potential for therapeutic intervention. Cancer Biol Ther, 2012. 13(7): p. 451-7.
- Adamia, S., et al., Combination therapy targeting Erk1/2 and CDK4/6i in relapsed refractory multiple myeloma. Leukemia, 2022.
- Jung, H., et al., Intron retention is a widespread mechanism of tumor-suppressor inactivation. Nat Genet, 2015. 47(11): p. 1242-8.
- Hsu, T.Y., et al., The spliceosome is a therapeutic vulnerability in MYC-driven cancer. Nature, 2015. 525(7569): p. 384-8.
Follow the Topic
-
Leukemia

This journal publishes high quality, peer reviewed research that covers all aspects of the research and treatment of leukemia and allied diseases. Topics of interest include oncogenes, growth factors, stem cells, leukemia genomics, cell cycle, signal transduction and molecular targets for therapy.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in