Do I drink your blood or not? A mosquito’s dilemma
Published in Microbiology and Zoology & Veterinary Science

All female mosquitoes bite and partake in blood meals to obtain the protein needed to lay eggs. In doing so, many mosquito species become vectors (carriers) of parasitic diseases. Aedes aegypti, for example, transmits the viruses that cause Zika, dengue, chikungunya, Mayaro and yellow fever (the mosquito is commonly referred to as the yellow fever mosquito). Other mosquitoes, such as Anopheles gambiae, transmit the parasite that causes malaria.
But why and how do mosquitoes home in on their blood meal and how do they decide if they should eat and how much? Well, apparently it is all in the blood!
In particular, it depends on the presence of certain molecules (phagostimulants) in the blood that can attract and promote the decision to take a blood meal. These molecules are called adenine nucleotides and are made of three components: a nitrogenous base, a 5-carbon sugar and phosphate groups. They are found in DNA (Adenine is one of the nucleotide bases making up the DNA double helix), are part of molecular signalling pathways, are used as biological energy currency within cells, and they are found in high concentrations in platelets in the blood.
Mosquitoes use three chemoreceptive cells in the labral apical sensilla to detect adenine nucleotide. Several studies have investigated the mechanisms behind phagostimulants and how different mosquitoes interact with these molecules.
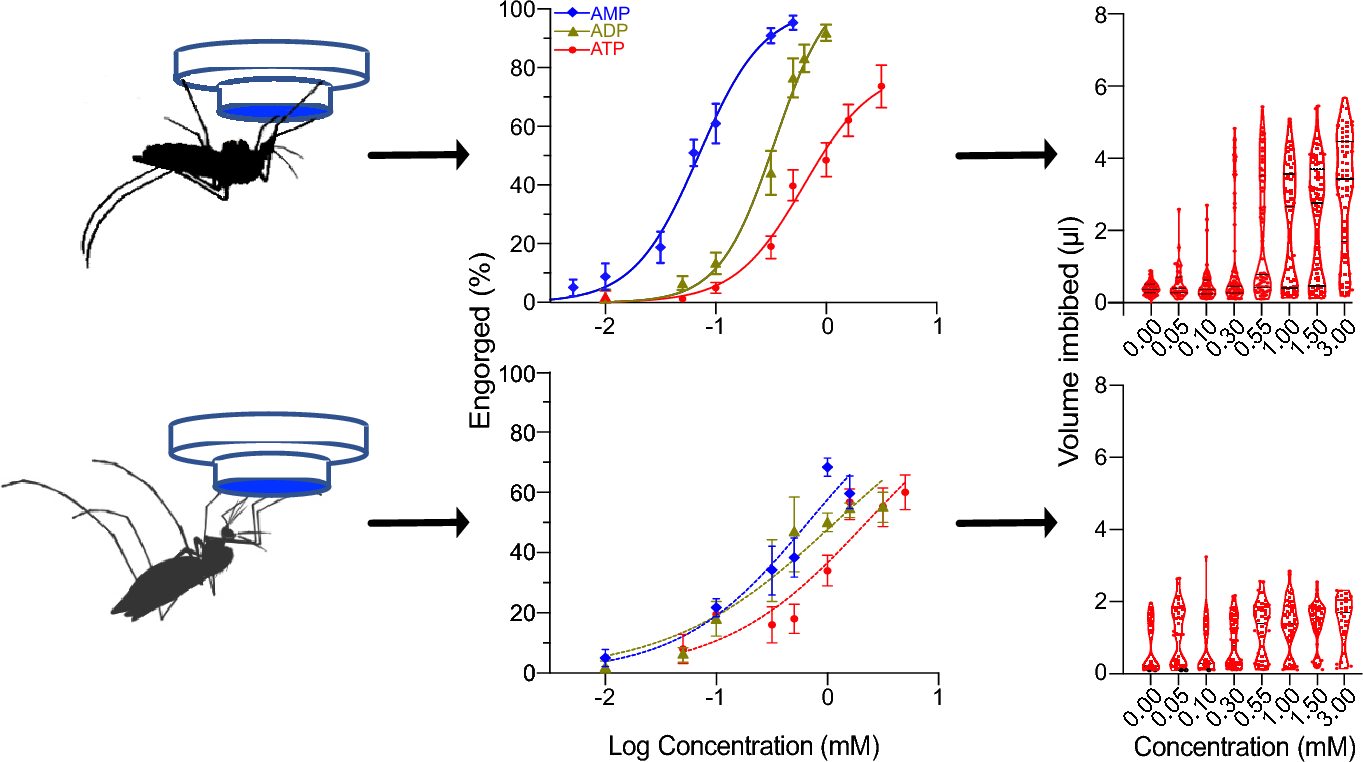
For example, it has been well established that Aedes aegypti require higher concentrations of the molecules to induce gorging. More recently, Matthew Mikenge and his team report in their paper in Parasites and Vectors their study of Aedes aegypti and Anopheles gambiae sensitivity to adenine nucleotides.
The team found that the sensitivity for adenine nucleotides differed between the two species, with Aedes aegypti being more sensitive to adenine nucleotides than Anopheles gambiae. However, the specificity for these molecules remained constant. This indicated that there is a species- specific tuning of the chemosensory neurons that respond to adenine nucleotides.
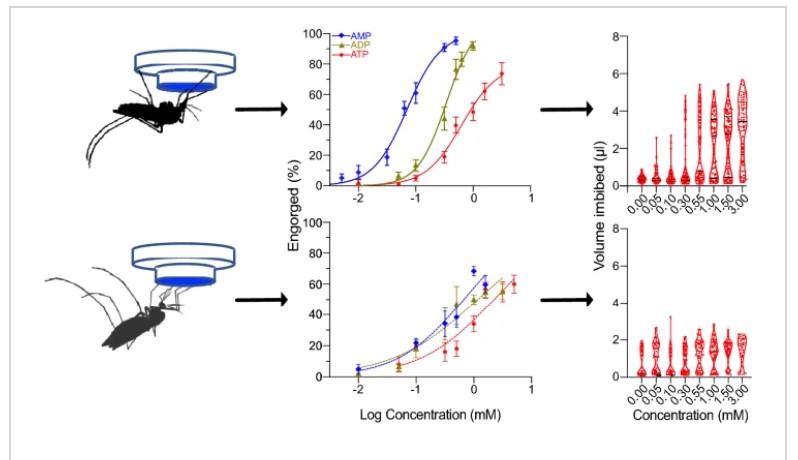
Furthermore, Anopheles gambiae could detect the carbonate buffer (negative control) in the absence of adenine nucleotides suggesting that it encodes blood phagostimulants in a different way to Aedes aegypti. Whilst Matthew Mikenge’s team did not delve further into what this difference could be, it is very much worth looking into for future studies.
Both mosquito species did display a dose dependent bi-modal (either not eating at all or gorging) corroborating what is already known. Also the high sensitivity to ATP (adenine triphosphate) of Ae. aegypti and An. gambiae supports the preference for ATP as a stimulant, which makes sense as ATP would be reliably available in the extracellular matrix upon rupture of epidermal and red blood cells.
Overall, the Matthew Mikenge and team provide useful insights into the sensitivity of mosquitoes to adenine nucleotides, that would form the groundwork for future in depth molecular and physiological studies.
Follow the Topic
-
Parasites & Vectors

This journal publishes articles on the biology of parasites, parasitic diseases, intermediate hosts, vectors and vector-borne pathogens.
-
BugBitten
A blog for the parasitology and vector biology community.
Related Collections
With Collections, you can get published faster and increase your visibility.
Credelio Quattro
Articles published in the collection have already gone through the systematic peer review process of the journal.
Publishing Model: Open Access
Deadline: Apr 24, 2026
Translational leishmaniasis
Topics of interest include:
• Analytical and omics-based advances for parasite detection, monitoring, and characterization.
• Comparative biology of Leishmania and related parasites, with emphasis on shared mechanisms of infection and survival.
• Host-pathogen interactions and immune responses.
• Epidemiology and transmission dynamics, including drawing parallels between leishmaniasis and other parasitic infections.
• Translational strategies in drug discovery, vaccine development, and therapeutic interventions applicable to leishmaniasis and related parasitic diseases.
By linking leishmaniasis with other parasitic diseases of similar characteristics, this collection seeks to foster integrative approaches, stimulate cross-disciplinary dialogue, and accelerate the translation of knowledge into innovative diagnostic, preventive, and therapeutic solutions.
Publishing Model: Open Access
Deadline: Jun 01, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in