First use of CRISPR-edited cells in a human patient
Published in Bioengineering & Biotechnology

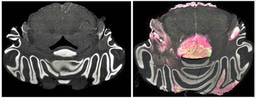
The team of Chinese oncologist You Lu, from Sichuan University in Chengdu, China, announced that a patient with aggressive lung cancer was treated last October with cells that were edited using CRISPR-Cas9 technology. The procedure was the first of its kind to use CRISPR (for ‘clustered regularly interspaced short palindromic repeats’), and joins transcription activator-like effector nucleases (TALEN) or zinc-finger nucleases (ZFN) as genome editing tools that have been employed therapeutically in humans.
Gene editing was first used therapeutically in humans in 2014, when scientists at the Perelman School of Medicine used ZFN-based genome editing to modify the CCR5 gene (a co-receptor for HIV entry) on T cells, which were injected in patients with AIDS to tackle HIV replication. Twelve patients with chronic HIV infection received autologous CD4 T cells carrying a ZFN-modified CCR5 gene; the procedure met the safety outcomes, and HIV DNA levels were decreased in most patients.
The second breakthrough in genome editing therapies was announced at the end of 2015. A team of immunologists at the Great Ormond Street Hospital for Children in London used TALEN-mediated gene editing to modify T cells from a healthy donor and treat a one-year-old patient with severe acute lymphoblastic leukemia. The procedure used ‘chimeric antigen receptor’ (CAR) T cells – T cells engineered to express chimeric receptors specific for a patient’s tumor.
As a genome engineering tool, CRISPR is considerably simpler, cheaper and more efficient than ZFNs or TALENs: whereas TALEN and ZFN proteins have to be engineered for each targeted DNA sequence, CRISPR-Cas9 relies on guide RNAs to target specific regions in the genome. The advantages of CRISPR over other genome engineering methods are such that TALENs and ZFNs are quickly being put aside in labs around the world.
The announcement that CRISPR-mediated immune cell editing was applied to treat lung cancer, besides revealing the first application of CRISPR-Cas9 in humans, also reinforces the idea that genome engineering could be particularly important in optimizing cell therapies. Because the gene editing procedure that modifies effector cells is done ex vivo, this protocol is simpler and carries fewer risks than, for instance, gene editing of tissues or cells inside the body. Nevertheless, several reports of in vivo CRISPR-based genome editing in animal models have been published, and studies are underway to optimize targeted delivery of the editing machinery and minimize off-target effects.
A clinical trial to study safety of T cell engineering using CRISPR-Cas9 for metastatic non-small lung cancer in Sichuan University (headed by Lu) is currently recruiting patients, and Peking University is also planning trials that use a similar ex vivo CRISPR-Cas9-based T-cell engineering approach to treat bladder, renal-cell and prostate cancers. Additional clinical trials to treat cancer and other human diseases are likely to be announced next year.
Further reading:
Urnov, F. D. et al. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 11, 636–646 (2010).
Joung, J. K. & Sander, J. D. TALENs: a widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 14, 49–55 (2013).
Doudna, J. A. & Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096 (2014).
Kim, J.-S. Genome editing comes of age. Nat. Protoc. 11, 1573–1578 (2016).
Tebas, P. et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N. Eng. J. Med. 370, 901–910 (2014).
Great Ormond Street Hospital for Children, NHS Foundation Trust. World first use of gene-edited immune cells to treat ‘incurable’ leukaemia (2015) Press release.
Banner image credit: Getty Images/iStockphoto Thinkstock Images \ Mark Gabrenya

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in