Giving the cell a rest - Targeting Cyclin D3 in B-cell acute lymphoblastic leukemia
Published in Cancer

Introduction
B-cell acute lymphoblastic leukemia (B-ALL) is the most common cancer among children with a second peak in incidence later in life. It is associated with good overall survival in pediatric patients (>90%) but dismal survival rates in adults (<40%) (WHO 2018).
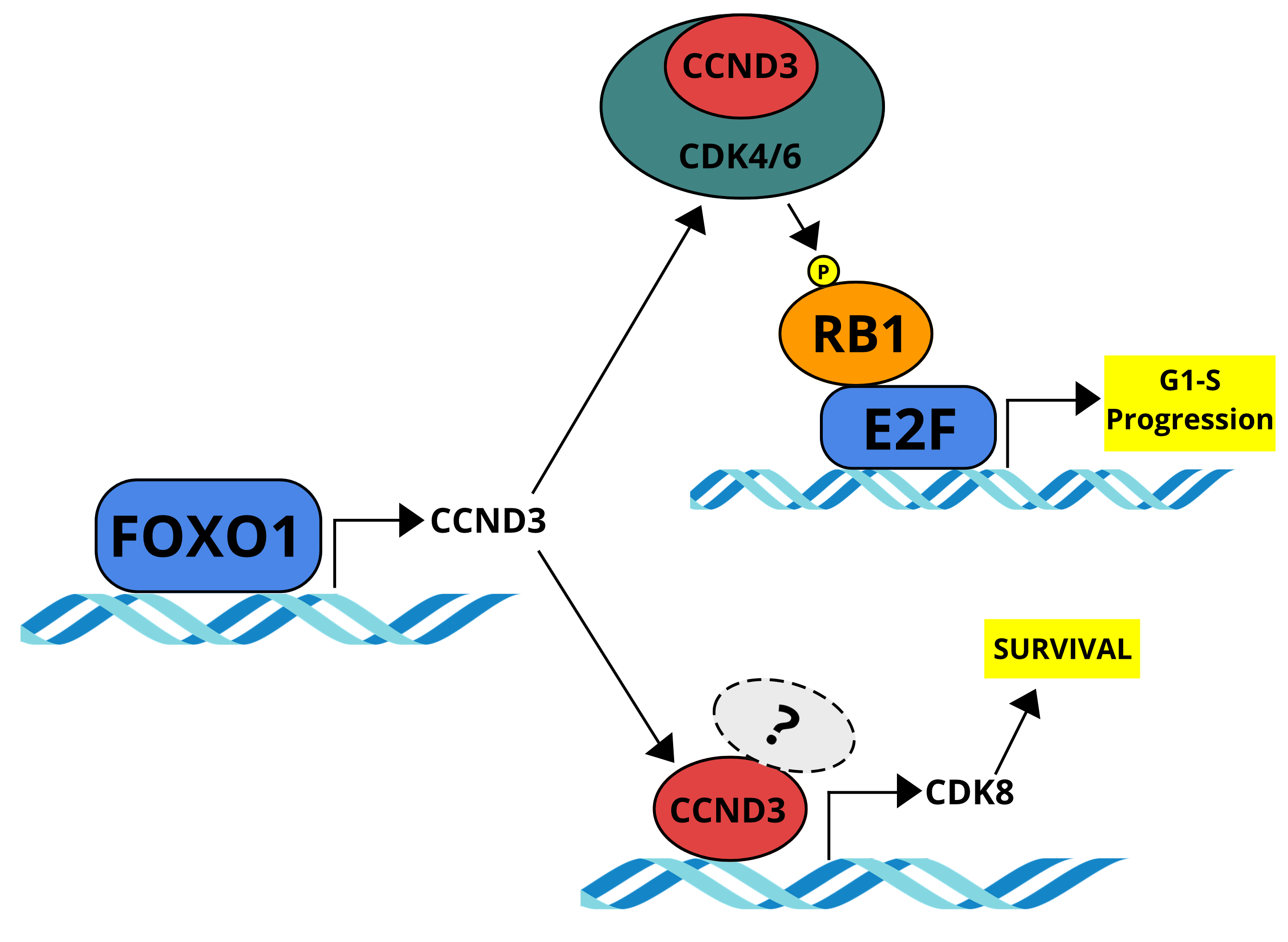
We have previously shown that FOXO1, a master transcription factor in B-cell development, is an essential factor for the survival of B-ALL cells and demonstrated the in vivo efficacy of a FOXO1 inhibitor in a patient-derived xenograft mouse model (Wang et al. 2018). Strikingly, the cytotoxic effects of genetic and pharmacological FOXO1 inhibition could be attenuated by the overexpression of Cyclin D3 (CCND3), which was down-regulated after FOXO1 depletion.
D-Cyclins (CCND1/2/3) as part of the CDK4/6-CCND holoenzyme are well-known drivers of cell cycle progression. Canonically, upon mitogenic stimulation, the CDK4/6-CCND complex phosphorylates RB1, the inhibitor of E2F. Following further phosphorylation by CDK2-CCNE, RB1 is released and degraded, thereby activating the G1-S transcriptional program through E2F.
Knock-out mice for the three D-Cyclins have been generated in the past, demonstrating partially overlapping functions, but also tissue-specificity, particularly for the need of CCND3 in the development of B-cells (Cooper et al. 2006). Furthermore, non-canonical roles of D-Cyclins, outside of cell cycle regulation and without CDK4/6, have been shown in the past, like modulation of the p53-pathway (Hydbring et al. 2016) or altering the accessibility of transcription factors to the chromatin by binding to the nuclear matrix (Karki et al. 2018). The role of CCND3 in B-ALL was never particularly analyzed, since CCND2, a canonical MYC-target has mostly been used as a surrogate for all three Cyclins in past functional studies and functional redundancy of the D-Cyclins had been widely assumed.
Based on our previous finding of FOXO1 knockdown-induced cytotoxicity being mediated by CCND3, we set to challenge the dogma of D-Cyclin redundancy in B-ALL. In addition, we wanted to understand how CCND3 expression is regulated by FOXO1, and unravel the anti-apoptotic, pro-survival mechanisms of CCND3 in B-ALL.
What we found:
What makes CCND3 so special?
Since B-ALL is a heterogeneous malignancy comprising multiple different genotypes, underlying driver mutations, and genetic programs, we analyzed publicly available, patient-derived RNA expression data as well as CRISPR/Cas9-knock-out screen data for the three D-Cyclins. RNA expression of CCND3 was the highest throughout all B-ALL groups analyzed. This was confirmed for human B-ALL cell lines of different genotypes. The knock-out screening data showed a genetic dependency of B-ALL cell lines only to CCND3.
This provided further incentive to look into what makes CCND3 so particularly important in B-ALL.
How are FOXO1 and CCND3 connected?
To understand why a gene is important, one must understand how it is regulated. Hence, we picked up where we left off at our last publication (Wang et al., 2018) and investigated how CCND3 expression is down-regulated after FOXO1 depletion. This time, we added an ex vivo murine Foxo1fl/fl BCR-ABL1-transformed B-ALL model to our experiments, for cleaner and faster Foxo1 knock-out compared to the previously used FOXO1-shRNAs in human cell lines. Confirming what we have previously observed, CCND3 is indeed the only D-type Cyclin down-regulated after FOXO1 depletion. By performing luciferase-assay, EMSA, and ChIP, we were able to reveal a previously unknown direct transcriptional activation of CCND3 by FOXO1 in B-ALL, finally uncovering the link between these two essential genes in the maintenance of B-cell lymphoblastic leukemia.
Removing only one of three D-Cyclins can't be too bad - right?
Functionally, we demonstrated that the depletion of CCND3 is cytotoxic and induces apoptosis in human cell lines, the BCR-ABL1 mouse model, and patient-derived xenografts with different underlying driver mutations. Although this is interesting by itself, it got even more intriguing when we compared the effects of CCND3 depletion to inhibition of its associated kinases CDK4/6 with palbociclib. In T-ALL, CCND3-CDK6 targeting by either shRNA or the CDK4/6 inhibitor palbociclib induced apoptosis by induction of reactive oxygen species (Wang et al. 2017). In B-ALL however, we observed only weak induction of apoptosis by treatment with palbociclib, particularly compared to the strong apoptosis after CCND3 depletion. Adding to the peculiar role of CCND3, its ectopic expression salvaged B-ALL cells from the growth-inhibitory effect induced by palbociclib. Additionally, CCND3 protein expression was highly increased by long-term exposure to palbociclib. Importantly, increased CCND3 expression was associated with decreased sensitivity to palbociclib. Nevertheless, the cells were still absolutely sensitive to CCND3 knockdown.
| Quick recap: CCND3 is highly expressed in B-ALL, its expression is activated by FOXO1, and its depletion but not inhibition of its associated kinases CDK4/6 induces apoptosis in B-ALL cells. |
But the question remained: What differentiates CCND3-knockdown from CDK4/6 inhibition?
To find an answer to this intriguing question, we compared the RNA expression profiles of three B-ALL cell lines with different underlying driver mutations after either treatment with palbociclib or shRNA-dependent knockdown of CCND3. As expected, many genes affected by both treatments overlapped. However, a small set of genes was exclusively regulated after CCND3 knockdown but not after CDK4/6 inhibition. This included the non-canonical CDK8, which depletion was recently shown to induce apoptosis in pre-clinical BCR-ABL1+ models of B-ALL (Menzl et al. 2019). We knocked down CDK8 in Ph+-like B-ALL and MLLr B-ALL, observing the same cytotoxic effect as previously described in BCR-ABL1+ models.
Why our findings are important:
Aberrant cell cycle regulation is a classical hallmark of cancer and a promising target for therapeutic intervention (Hanahan & Weinberg, 2011). Since the D-Cyclins were regarded as redundant and mere activators of CDK4/6 in B-ALL, our findings provide not only an intriguing insight into fundamental B-cell leukemia biology but also help us understand that targeting cell cycle components might be more complex than we originally thought. Although palbociclib is efficient even as monotherapy in breast cancer (Malorni et al. 2018) and pre-clinical T-ALL models (Wang et al. 2017), our study shows that B-ALL might be a different beast. Consequently, the degradation of CCND3 by PROTACs might be a better therapeutic strategy than inhibition of enzymatic activity of the CCND-CDK4/6 complex. Similarly, it has been shown that PROTAC-dependent degradation of CDK6 appeared more efficient than inhibition of enzymatic activity for induction of apoptosis in B-ALL (deDominici et al. 2020). Furthermore, our study elucidated one aspect of FOXO1-depletion-associated cytotoxicity through direct transcriptional control over CCND3 expression. This underlines the importance of FOXO1 for B-ALL survival and potentially paves the way for the development of a new generation of FOXO1 inhibitors or degraders.
Finally, we showed that CDK8 expression is modulated by CCND3 and that CDK8 is an essential gene not only in BCR-ABL1+ cases but also in B-ALL cells carrying other common mutations.
References
- Ketzer F, Abdelrasoul H, Vogel M, Marienfeld R, Müschen M, Jumaa H, et al. CCND3 is indispensable for the maintenance of B-cell acute lymphoblastic leukemia. Oncog 2022 111 [Internet]. 2022 Jan 10 [cited 2022 Jan 11];11(1):1–12. Available from: https://www.nature.com/articles/s41389-021-00377-0
- WHO. Cancer [Internet]. 2018. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer
- Wang F, Demir S, Gehringer F, Osswald CD, Seyfried F, Enzenmüller S, et al. Tight regulation of FOXO1 is essential for maintenance of B-cell precursor acute lymphoblastic leukemia. Blood. 2018 Jun;131(26):2929–42.
- Hydbring P, Malumbres M, Sicinski P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat Rev Mol Cell Biol [Internet]. 2016 Apr 1;17(5):280–92. Available from: https://doi.org/10.1038/nrm.2016.27
- Karki S, Kennedy DE, Mclean K, Grzybowski AT, Maienschein-Cline M, Banerjee S, et al. Regulated Capture of Vκ Gene Topologically Associating Domains by Transcription Factories. Cell Rep [Internet]. 2018 Aug 28 [cited 2020 Jan 29];24(9):2443–56. Available from: https://www.sciencedirect.com/science/article/pii/S2211124718312245
- Cooper AB, Sawai CM, Sicinska E, Powers SE, Sicinski P, Clark MR, et al. A unique function for cyclin D3 in early B cell development. Nat Immunol [Internet]. 2006;7(5):489–97. Available from: https://doi.org/10.1038/ni1324
- Wang H, Nicolay BN, Chick JM, Gao X, Geng Y, Ren H, et al. The metabolic function of cyclin D3-CDK6 kinase in cancer cell survival. Nature [Internet]. 2017/06/07. 2017 Jun 15;546(7658):426–30. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28607489
- Menzl I, Zhang T, Berger-Becvar A, Grausenburger R, Heller G, Prchal-Murphy M, et al. A kinase-independent role for CDK8 in BCR-ABL1+ leukemia. Nat Commun [Internet]. 2019;10(1):4741. Available from: https://doi.org/10.1038/s41467-019-12656-x
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Vol. 144, Cell. Cell Press; 2011. p. 646–74.
- Malorni L, Curigliano G, Minisini AM, Cinieri S, Tondini CA, D’Hollander K, et al. Palbociclib as single agent or in combination with the endocrine therapy received before disease progression for estrogen receptor-positive, HER2-negative metastatic breast cancer: TREnd trial. Ann Oncol Off J Eur Soc Med Oncol [Internet]. 2018 Aug 1 [cited 2022 Jan 11];29(8):1748–54. Available from: https://pubmed.ncbi.nlm.nih.gov/29893790/
- De Dominici M, Porazzi P, Xiao Y, Chao A, Tang HY, Kumar G, et al. Selective inhibition of Ph-positive ALL cell growth through kinase-dependent and -independent effects by CDK6-specific PROTACs. Blood [Internet]. 2020 Apr 30 [cited 2021 May 6];135(18):1560–73. Available from: http://ashpublications.org/blood/article-pdf/135/18/1560/1725347/bloodbld2019003604.pdf
Banner picture modified from: https://commons.wikimedia.org/wiki/File:Bicycle.svg
Follow the Topic
-
Oncogenesis

A peer-reviewed open access online journal that publishes articles exploring mechanistic insight and molecular basis of cancer and related phenomena. It seeks to promote diverse and integrated areas of molecular biology, cell biology, oncology, and genetics.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in