Coordinate transcriptional regulation of ErbB2/3 by C-terminal binding protein 2 signals sensitivity to ErbB2 inhibition in pancreatic adenocarcinoma
Published in Cancer
Background:
Previously, we reported overexpression of oncoprotein C-terminal Binding Proteins (CtBP) 1 and 2 in human Pancreatic Ductal Adenocarcinoma (PDAC) patients and demonstrated allelic loss of CtBP2 results in decreased overall tumor burden and prolonged survival in the transgenic CKP (Pdx1.Cre; LSL.K-RasG12D/+; p53fl/fl) mouse model1. In this paper, we attempted to further explore the mechanistic role of CtBP2 in PDAC tumorigenesis and discovered an intriguing and therapeutically impactful link between CtBP2 and ErbB3 (a member of the EGFR family of receptors) possibly regulating PDAC growth and metastasis.
What did we do:
We generated a cell line from CKP mouse primary PDAC tumors and knocked out CtBP2 using CRISPR-Cas9 to generate CKP/KO cells. Upon orthotopic injection of CKP and CKP/KO cells into immunodeficient mouse pancreata, CKP/KO cells formed smaller tumors with vastly lower rate of peritoneal metastasis, and those mice survived longer compared to the mice injected with CKP cells. Upon transcriptomic profiling of the tumors from both cohorts (CKP and CKP/KO), we found significant downregulation of ErbB3 expression in CKP/KO tumors compared to CKP tumors. Moreover, upon further exploration of expression of EGFR family members in CKP vs. CKP/KO cells, we noted coordinate down regulation of ErbB2, but not ErbB1/EGFR, protein and mRNA in CKP/KO vs. CKP cells. Though ErbB3 has been previously linked to poor outcomes in pancreatic cancer2, the mechanism of regulation of both ErbB2 and ErbB3 expression, outside of somatic ErbB2 gene amplification, has remained obscure, thus our study is the first to link an oncogenic epigenetic regulator (CtBP) with control of ErbB2/3 expression.
Next, we validated the functional significance of the novel connection of CtBP to ErbB3 by observing the activity of growth factor signaling pathways upon treatment of CKP vs. CKP/KO cells with the ErbB3 ligand Neuregulin-1 (NRG-1). Upon serum starvation and incubation of cells with NRG-1, CKP cells with high ErbB3 demonstrated increased p-ErbB3, as well as activated downstream PI3K and MAPK pathways evidenced by increased p-Akt and p-Erk1/2, while NRG-1 downstream signaling in CKP/KO cells with low ErbB3 was nearly completely abrogated with little or no increase in p-ErbB3, p-Akt, or p-Erk1/2.
We next interrogated the CtBP2/ErbB3 connection and phenotypic influence of ErbB3 expression in a human PDAC cell line (HPAC). Indeed, stable knockdown of CtBP2 decreased ErbB2/3 levels exactly as seen in the mouse CKP/KO cells, and we observed that among 8 human PDAC cell lines, 4 demonstrated robust ErbB3 expression and 4 exhibited barely detectable or no ErbB3 expression, aligned with limited clinical studies showing that 30-60% of PDAC demonstrate high levels of ErbB3 by immunohistochemistry3,4. Functionally, stable knockdown of ErbB3 in HPAC cells strongly attenuated growth in culture, suggesting ErbB3-expressing PDAC cells are addicted to ErbB3 expression2.
Based on these data, we hypothesized that PDAC cells with high level ErbB3 expression were dependent on growth signaling from ErbB3 in complex with its heterodimeric partner ErbB2. Thus, we interrogated the translational relevance of our findings, noting that sensitivity to the ErbB2-targeted tyrosine kinase inhibitor (TKI) lapatinib was correlated directly with ErBb3 expression levels in the panel of 9 (8 human + mouse CKP) PDAC cell lines (Fig 1).
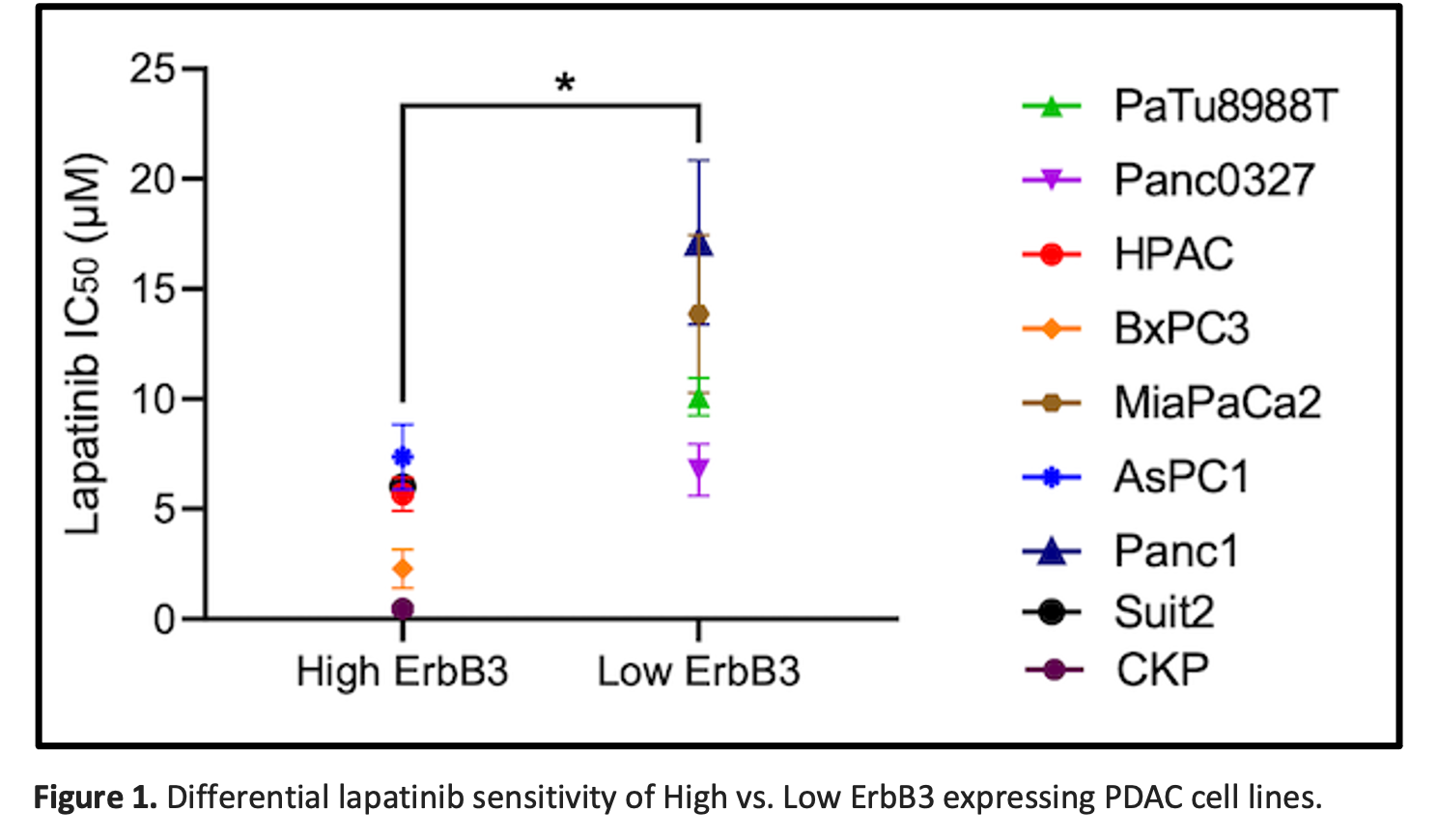
Conclusions:
Overall, in this paper, we have shown that CtBP2-dependent PDAC tumorigenesis is regulated via ErbB2/3 signaling, have highlighted a critical mechanistic role of ErbB3 in PDAC, and demonstrated how ErbB3 levels can affect PDAC cell sensitivity to an ErbB2-targeted TKI.
Our work highlights the potential importance of ErbB2/ErbB3 heterodimeric complexes as therapeutic targets in PDAC that express high levels of ErbB3 (30-60% of PDAC)3,4 in a setting of non-amplified physiologic ErbB2 expression (Fig. 2). Though our work tested an earlier generation TKI (lapatinib) as proof of principle, our ongoing work will determine if current highly effective anti-ErbB2 therapies such as the trastuzumab/tucatinib combination5, biologic agents that directly target ErbB36, or emerging autologous T-cell therapies targeting ErbB2/3 in solid tumors7 will be effective as precision therapeutic strategies in ErbB3-expressing PDAC.
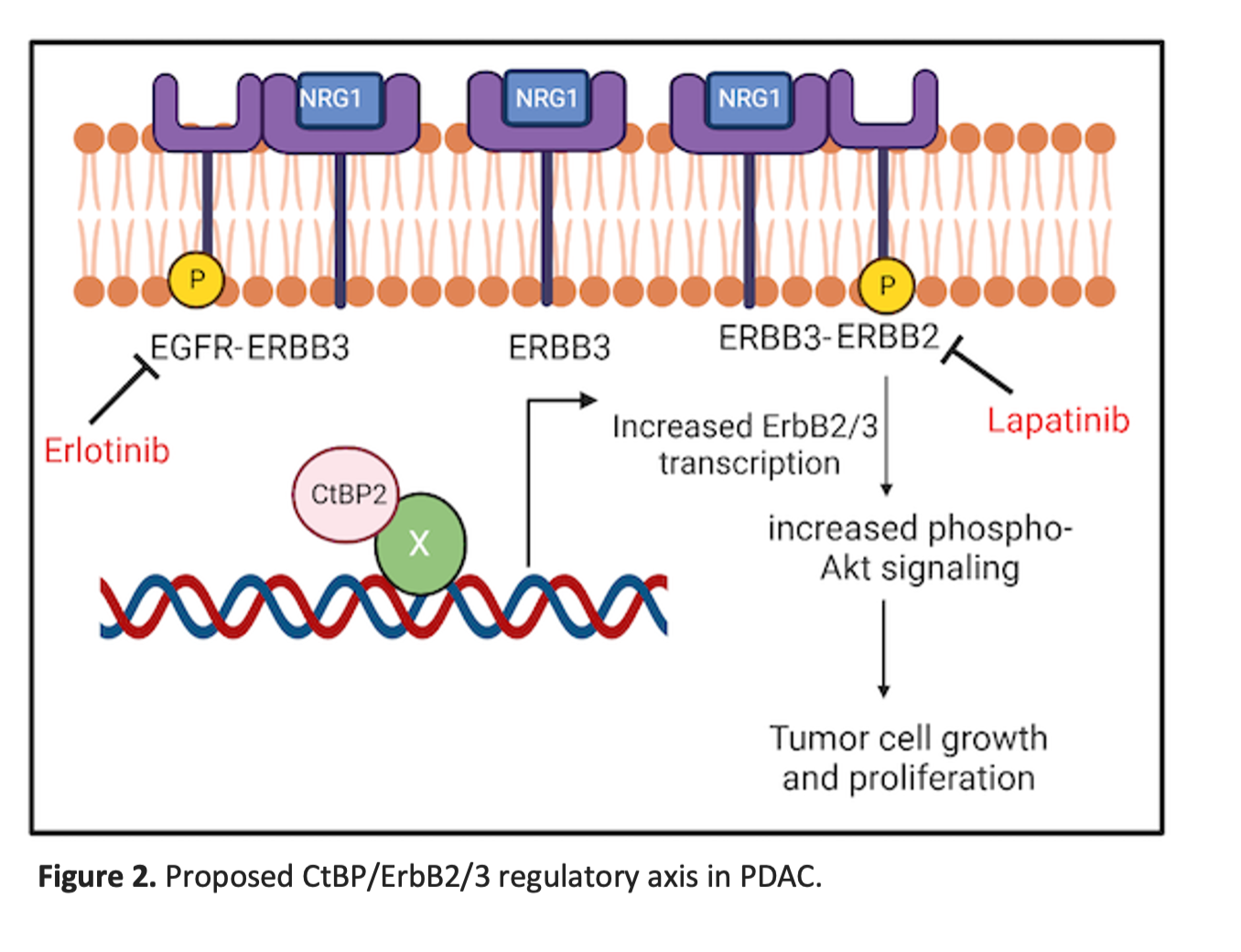
References:
- Chawla AT, Chougoni KK, Joshi PJ, Cororaton AD, Memari P, Stansfield JC, et al. CtBP—a targetable dependency for tumor-initiating cell activity and metastasis in pancreatic adenocarcinoma. Oncogenesis. 2019;8:55.
- Liles JS, Arnoletti JP, Tzeng C-WD, Howard JH, Kossenkov AV, Kulesza P, et al. ErbB3 expression promotes tumorigenesis in pancreatic adenocarcinoma. Cancer Biol Ther. 2010;10:555–63.
- Thomas G, ChardèS T, Gaborit N, Mollevi C, Leconet W, Robert B, et al. HER3 as biomarker and therapeutic target in pancreatic cancer: new insights in pertuzumab therapy in preclinical models. Oncotarget. 2014;5:7138–48.
- Desai O, Wang R. HER3- A key survival pathway and an emerging therapeutic target in metastatic colorectal cancer and pancreatic ductal adenocarcinoma. Oncotarget. 2023;14:439-443.
- Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N Engl J Med. 2020;382:597-609.
- Majumder A. HER3: Toward the Prognostic Significance, Therapeutic Potential, Current Challenges, and Future Therapeutics in Different Types of Cancer. Cells. 2023;12:2517.
- Maennling AE, Tur MK, Niebert M, Klockenbring T, Zeppernick F, Gattenlöhner S, et al. Molecular Targeting Therapy against EGFR Family in Breast Cancer: Progress and Future Potentials. Cancers (Basel). 2019;11:1826.
Follow the Topic
-
Oncogenesis

A peer-reviewed open access online journal that publishes articles exploring mechanistic insight and molecular basis of cancer and related phenomena. It seeks to promote diverse and integrated areas of molecular biology, cell biology, oncology, and genetics.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in