Glioblastoma chemoresistance driven by PI3K signaling and channel protein Cx43
Published in Cancer

Explore the Research

Connexin 43 confers chemoresistance through activating PI3K - Oncogenesis
Oncogenesis - Connexin 43 confers chemoresistance through activating PI3K
Glioblastoma (GBM) is the most common central nervous system malignancy, representing 48% of malignant brain tumors. GBM has no cure, and the few treatment options available provide only mild improvements in a clinical setting. Though GBM research continues to advance, the year-to-year changes in prognosis remain stagnant, with a 5-year survival rate of only 6.8% and an average survival length of 14.6 months1, 2. To make matters worse, GBM inevitably recurs within two years, and patients with recurrent disease have an average survival of only 5.5-7.5 months3. Upon recurrence, the tumor is resistant to current GBM therapies, including chemotherapy, effectively leaving no further therapeutic options. Therefore, it is essential to find ways to overcome this resistance in order to effectively improve GBM outcomes.
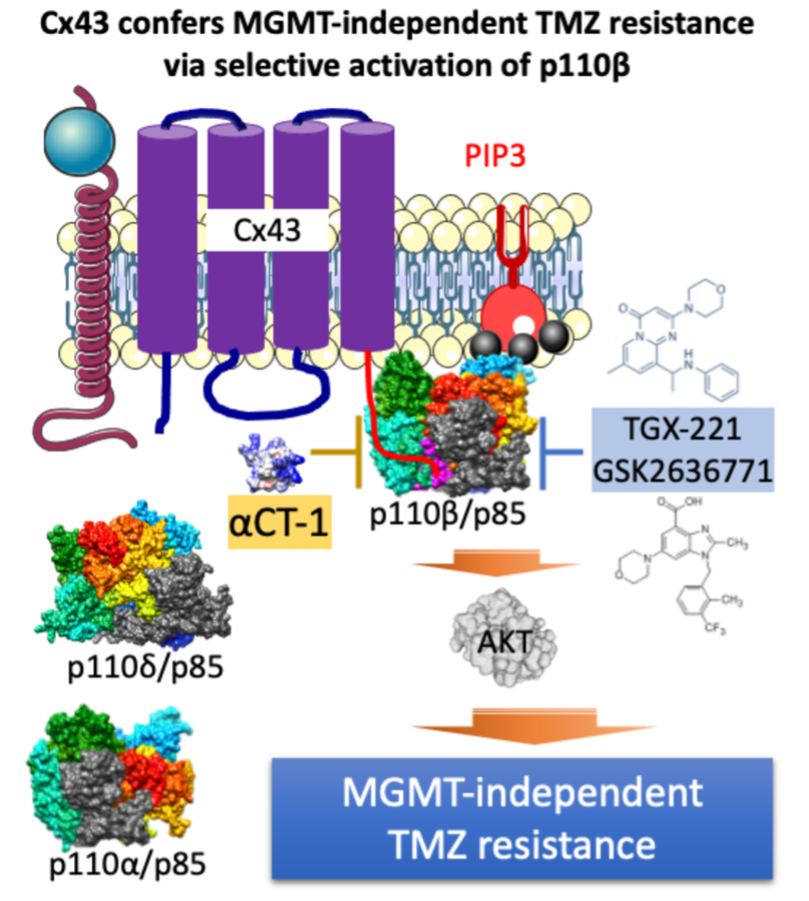
In our recent publication in Oncogenesis4, we approached this problem by exploring the therapeutic potential of the gap junction protein, connexin 43 (Cx43), which has been shown in previous studies to control chemotherapy responsiveness in GBM5, 6. Through analysis of data obtained from multiple publicly available databases, we found that levels of Cx43 mRNA, but not other connexins, could predict GBM response to chemotherapies and the risk of poor prognosis. However, how exactly a membrane channel protein could render brain cancer cells refractory to chemotherapies remained elusive, making it difficult to develop a Cx43-based treatment to effectively treat drug-resistant GBM.
To resolve this issue, we explored several signaling pathways previously shown to act downstream of Cx43 and found that the phosphatidylinositol-3 kinase (PI3K) pathway may be responsible for Cx43-induced chemoresistance. We discovered that αCT17, a Cx43 peptide inhibitor, induces a significant decrease in downstream PI3K activity in chemoresistant GBM cell lines. To further support our findings, we employed RNA interference techniques to ablate expression of the Cx43 gene in Cx43-high GBM cells and, reciprocally, overexpress Cx43 in chemotherapy-sensitive Cx43-low GBM cell lines. As expected, knockdown of Cx43 mitigated PI3K activity, whereas overexpression of Cx43 upregulated this signaling pathway. Next, we tested to determine if the Cx43 membrane channels are essential for activating PI3K. Small molecules such as ATP and glutamate can be released from these channels and serve as PI3K activators. Surprisingly, in drug-resistant GBM cells, levels of ATP and glutamate remained unchanged or even increased following the inhibitory action of αCT1, showing that Cx43 utilizes a different mechanism in PI3K activation.
Inspired by the finding that the Cx43 C-terminal tail physically interacts with signaling molecules independent of its channel activity, we tested whether Cx43 directly binds to PI3K proteins to activate this signaling. The PI3K family is comprised of four catalytic subunits: p110α, p110β, p110δ, and p110γ. Our lab has recently shown that catalytic subunits are not functionally redundant and that the β isoform is the most dominant in chemoresistant GBM8. To test whether these subunits were functionally divergent in Cx43-induced activation, we analyzed protein expression taken from six different GBM cell lines. We found a significant positive correlation between Cx43 levels and p110β but no association among the other PI3K catalytic or regulatory subunits. In addition, a positive correlation was found between mRNA expression of p110β and Cx43 but not with other connexin variants. To test for a direct interaction, we performed co-precipitation experiments and, consistent with our expectation, Cx43 interacted with p110β but not p110α or p110δ. αCT1, which has the same amino acid sequence as the Cx43 C-terminus, interfered with this binding.
These findings led us to design a triple combination therapy to target the connection between Cx43 and PI3K catalytic subunit p110β. We theorized that targeting both Cx43 and p110β would lead to significant perturbation of PI3K and synergistic sensitization to the chemotherapy temozolomide (TMZ). We took newly dissected primary GBM cells, GBM cell lines, and GBM stem cells and dosed them with various combinations of αCT1, TMZ, and one of two clinically tested p110β inhibitors (TGX-221 or GSK2636771). GBM cells that expressed high levels of Cx43 and p110β experienced a significant decrease in viability when treated with this triple combination. Bliss independence model analyses showed significant synergistic effects for triple therapy combinations in TMZ-resistant GBM cell lines. Identical treatment in non-pathological human astrocytes suggests that our drug combinations do not exacerbate non-selective TMZ toxicity in the brain. Collectively, our data demonstrate that simultaneous inhibition of Cx43 and p110β is an effective approach for overcoming MGMT-independent TMZ resistance in GBM.
All in all, we found that Cx43 activates PI3K independent of Cx43 channels and identified a pre-clinical triple combination therapy that targets Cx43 and PI3K to overcome chemoresistance in GBM (Figure 1). We are optimistic about future research stemming from this work and believe our findings, especially the triple combination therapy, will directly benefit GBM patients.
- Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro Oncol. Oct 1 2018;20(suppl_4):iv1-iv86. doi:10.1093/neuonc/noy131
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. Jan 2019;69(1):7-34. doi:10.3322/caac.21551
- Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma--are we there yet? Neuro Oncol. Jan 2013;15(1):4-27. doi:10.1093/neuonc/nos273
- Pridham KJ, Shah F, Hutchings KR, et al. Connexin 43 confers chemoresistance through activating PI3K. Oncogenesis. Jan 12 2022;11(1):2. doi:10.1038/s41389-022-00378-7
- Grek CL, Sheng Z, Naus CC, Sin WC, Gourdie RG, Ghatnekar GG. Novel approach to temozolomide resistance in malignant glioma: connexin43-directed therapeutics. Curr Opin Pharmacol. Aug 2018;41:79-88. doi:10.1016/j.coph.2018.05.002
- Murphy SF, Varghese RT, Lamouille S, et al. Connexin 43 Inhibition Sensitizes Chemoresistant Glioblastoma Cells to Temozolomide. Cancer Res. Jan 1 2016;76(1):139-49. doi:10.1158/0008-5472.CAN-15-1286
- O'Quinn MP, Palatinus JA, Harris BS, Hewett KW, Gourdie RG. A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia following ventricular injury. Circ Res. Mar 18 2011;108(6):704-15. doi:10.1161/CIRCRESAHA.110.235747
- Pridham KJ, Le L, Guo S, et al. PIK3CB/p110beta is a selective survival factor for glioblastoma. Neuro Oncol. Mar 27 2018;20(4):494-505. doi:10.1093/neuonc/nox181
Follow the Topic
-
Oncogenesis

A peer-reviewed open access online journal that publishes articles exploring mechanistic insight and molecular basis of cancer and related phenomena. It seeks to promote diverse and integrated areas of molecular biology, cell biology, oncology, and genetics.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in