Heteroatom‑Coordinated Fe–N4 Catalysts for Enhanced Oxygen Reduction in Alkaline Seawater Zinc‑Air Batteries
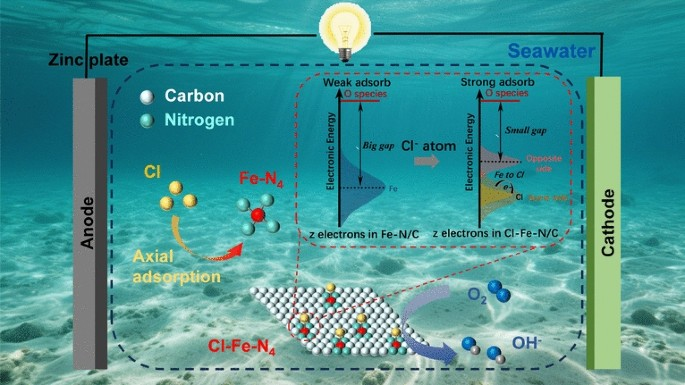
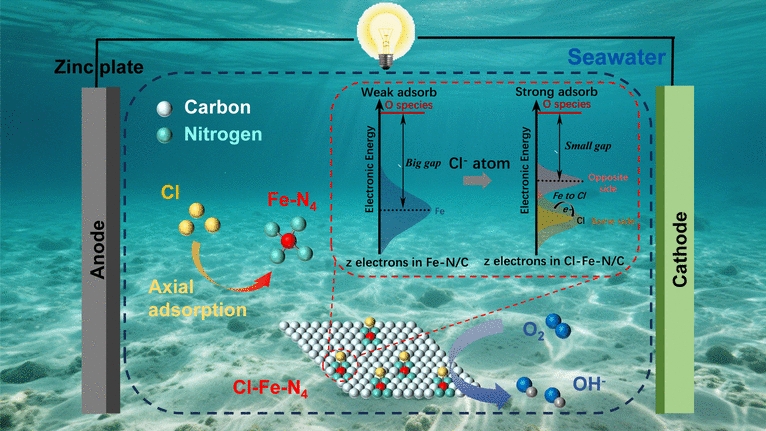
As maritime electrification and blue-energy harvesting accelerate, conventional Pt/C cathodes collapse in natural seawater because chloride ions poison active sites and shift the oxygen-reduction pathway from the desired 4 e- route to the parasitic 2 e- peroxide route. Now, researchers from Central South University and Xi’an Jiaotong-Liverpool University, led by Professor Jun Wu and Professor Danlei Li, have unveiled a universal oxidative-polymerization route that axially clamps Fe–N4 single-atom sites with heteroatoms (Cl or S) to create square-pyramidal “Cl–Fe–N4” catalysts that repel Cl- while doubling reaction kinetics. The work is published in Nano-Micro Letters.
Why Heteroatom Axial Coordination Matters
• Chloride Shield: Axial Cl pulls electron density toward itself, generating a negatively charged Fe center that electro-statically rejects Cl⁻ adsorption, keeping 96.7 % current retention after 12 h in pH-13 synthetic seawater.
• Intrinsic Boost: The Cl–Fe bond shortens the Fe–N bond length to 1.91 Å, down-shifts the d-band center to –2.94 eV and lowers the *OH-to-H2O rate-limiting step from 0.86 eV to 0.63 eV, delivering a record 5.8 mA cm-2 limiting current density that outperforms 40 wt % Pt/C (3.0 mA cm-2).
• Seawater Compatibility: The five-coordinated geometry maintains a strict 4 e- pathway (n = 4.02) even in 0.5 M KCl, whereas undoped Fe–N4 collapses to 2.24.
Innovative Design and Features
• One-Pot Synthesis: 1,5-diaminonaphthalene oxidative polymerization at 80 °C followed by 950 °C Ar pyrolysis and acid leaching yields 708 m2 g-1 microporous carbon with atomically dispersed Fe and 4.73 % N content.
• Spectroscopic Proof: HAADF-STEM, XANES and EXAFS confirm the absence of Fe clusters; only a single Fe–Cl shell at 2.17 Å is detected, matching square-pyramidal Cl–Fe–N4.
• Device Validation: When coated on nickel-foam air-cathodes (1 mg cm-2), the Cl–Fe–N4 seawater zinc-air battery delivers 187.7 mW cm-2 peak power at 245.1 mA cm-2 and sustains 200 h of deep discharge–charge cycles at 10 mA cm-2 with only 7 mV half-wave-potential decay.
Applications and Future Outlook
• Maritime Power Packs: Coupled with printable Zn anodes, the catalyst enables pouch cells that operate directly in ocean water, promising buoy-based sensors and unmanned underwater vehicles.
• Off-Grid Desalination: High current density at low overpotential allows SZAB-driven membrane pumps that consume 30 % less energy than conventional reverse-osmosis systems.
• Scalable Manufacturing: The precursor ink is water/ethanol-based and the maximum processing temperature is <1000 °C, making roll-to-roll production compatible with existing carbon-fiber lines.
• Next-Gen Tuning: The team is exploring F and Br axial ligands and dual-metal (Fe/Co) centers to further widen the operating salinity window to 10 wt % NaCl.
This comprehensive study provides a materials-by-design playbook for turning the most abundant anion in the ocean—chloride—from a poison into a performance descriptor, paving the way for truly seawater-robust energy storage and conversion devices.
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in