Dormancy refers to a state of suspended growth in plants, a protective strategy for them to survive harsh weather conditions. Our team has been studying dormancy in geophyte plants for over 30 years, exploring the physiological mechanisms involved in dormancy and methods for dormancy release. The process of bud-growth transition (BGT) in Gladiolus croms, from dormancy to dormancy release, is regulated by external environmental factors and endogenous signals. Hormone and carbohydrate levels exhibit regular changes and play important roles. Interestingly, our team innovatively discovered a new treatment that promotes BGT: wound. This phenomenon is not only observed in gladiolus but also occurs in bulb crops such as onions and garlic. This prompts us to further investigate the underlying mechanisms behind the wound.
From a Simple Wound to Scientific Questions
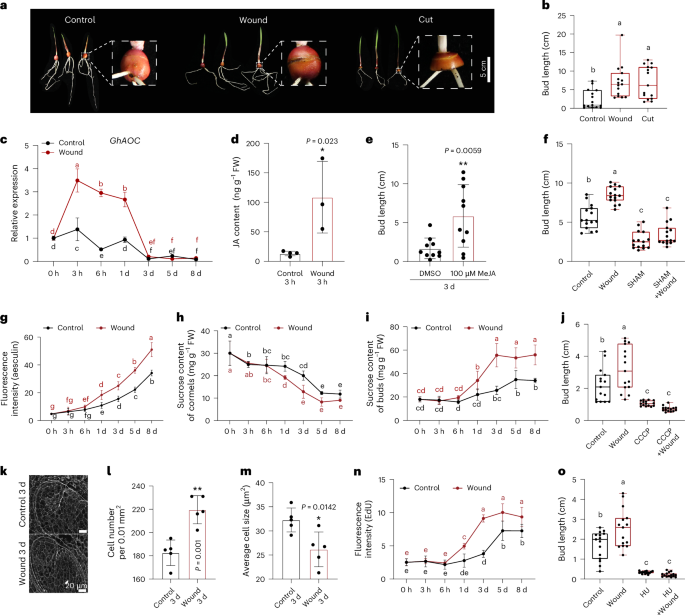
This project began in 2011. We experimented with cutting off most of the corm and leaving only the bud and a small portion of the corm for planting and cutting off the lower half of the corm for planting, then observing BGT. The results showed that cutting significantly promoted BGT. However, the removal of most of the corms resulted in large wound areas. Since the preliminary experiments were conducted under tissue culture conditions, these wounds caused severe bacterial and fungal infections, leading to a significant rise in contamination. Therefore, this prompted us to further explore new damage methods and processing conditions. In field trials, we applied a circular incision treatment that caused less wounding to the corms and resulted in less nutrient loss, finding that circular incision significantly increased the BGT. Therefore, we further conducted multiple experiments in the laboratory under different conditions (tissue culture conditions, soil planting, and glass culture bottles with cotton) to investigate the effect of wounding on BGT, ultimately developing a highly consistent, operable, and low-contamination circular incision treatment method.
After we discovered that wound can promote BGT, a more fundamental question emerged: How exactly do wound signals gradually "awaken" dormant buds? What kind of molecular dialogue lies behind this? We selected cormels with clearly defined phenotypes 8 days after treatment (after 8 days of wounding, 64% of the cormels had apical buds break through the tunic, while only 38% of the controls had sprouted) for transcriptome sequencing analysis, thereby discovering the potential roles of jasmonic acid (JA), sugars, and cell division in wound-induced BGT. However, because the plant's response to wounding is very rapid, we must begin tracking relevant indicators shortly after wounding. Therefore, we measured the transcriptional level of JA, apoplastic sugar transport capacity, sucrose content in corms and buds, and cell division activity within 0-8 days after wounding, thereby we have pioneered the discovery of a molecular pathway that links wound signals to the BGT.
Connecting the Dots: JA, Sugar, and the Cell Division Cascade
In order to clarify the relationships among wound, jasmonic acid, sugar transport and cell division, we designed a series of "intervention" experiments: we applied jasmonic acid externally to the cormels, applied sucrose externally, or used inhibitors to block jasmonic acid synthesis, sucrose transport or cell cycle. We wanted to see if the promoting effect of wound on BGT would still occur if any of these steps were "switched off" at some point. The results are very interesting. All these inhibitors can block the BGT induced by wounding. This means that jasmonic acid, sucrose transport, and cell division are indeed the key "stations" on this pathway. Further tests have shown that the jasmonic acid produced by wound can enhance the transport of sucrose from the cormels to the buds; and the accumulated sugar in the bud further activates cell division. A clear signal chain gradually emerges: wound - jasmonic acid - sucrose transport - cell division – BGT.
So, which genes are responsible for these functions? We selected two candidate genes from the transcriptome data: GhSUT4 (which is involved in sucrose transport) and GhCYCD2;1 (which regulates the cell cycle). Both of them were strongly induced under wound and jasmonic acid. This led us to believe that they are likely the key executors connecting the signals with the functions. Finally, we also wanted to know who is directing the expression of these two genes. Through yeast one-hybrid screening, we identified a key transcription factor: GhZAT11. It is also induced by wound and jasmonic acid and can directly bind to the promoters of GhSUT4 and GhCYCD2;1, activating their transcription. Thus, a complete signaling pathway has finally been linked together: wound - jasmonic acid - GhZAT11 - (GhSUT4 / GhCYCD2;1) - sugar transport and cell division - BGT.
A Conserved Survival Strategy Across Species
After clarifying this pathway in the Gladiolus, we began to wonder: is this "wound-induced BGT" strategy unique to the Gladiolus, or is it a common conservative survival wisdom that exists in a wider range of plants? To answer this question, we turned our attention to other bulbous crops that also have dormancy characteristics - garlic and onion. We processed their bulbs using the same circular cutting method and observed their BGT. The results were encouraging: the wound also significantly promoted the BGT of garlic and onions. This suggests that this response is not accidental but rather a common biological strategy across species.
Starting from a Gladiolus, and moving on to garlic and onion, we have witnessed the commonality of natural strategies. Scientific exploration often begins with an exceptional case of a species, but ultimately leads to more universal laws of the life world.
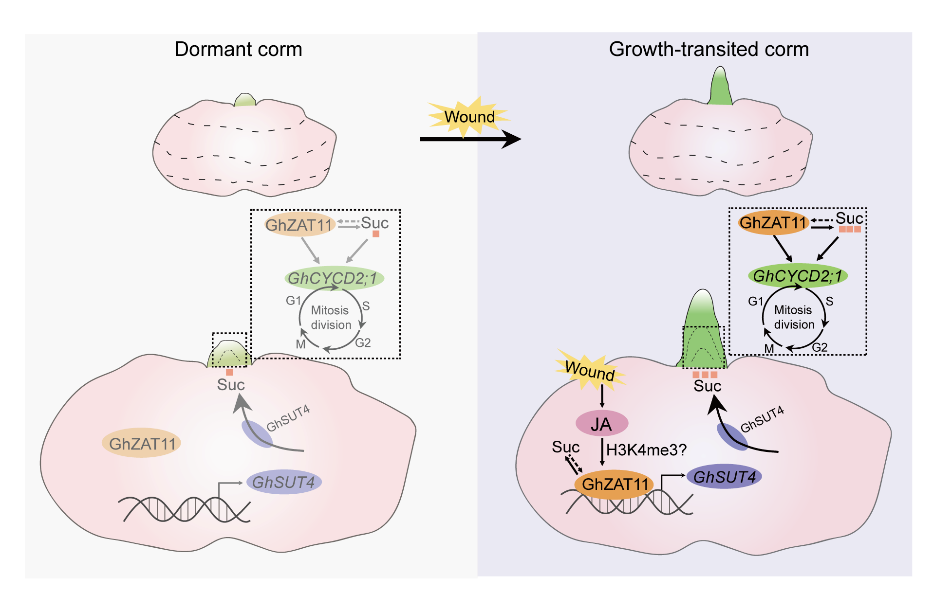
Figure 1 Schematic diagram of wounding-induced bud-growth transition. Upon wounding, dormant cormels exhibit a rapid surge in JA levels, which likely triggers GhZAT11 expression via H3K4me3 epigenetic regulation. Elevated GhZAT11, in turn, drives the expression of the sucrose transporter gene GhSUT4, facilitating the apoplastic transport of sucrose from the corm to the bud to support energy demands. Concurrently, GhZAT11 activates the cell cyclin gene GhCYCD2;1, promoting cell division and accelerating the bud-growth transition (BGT).
From Insight to Application: Implications of the Wake-Up Call
This study not only elucidates the molecular mechanism by which wounding promotes bud growth transition (BGT) through the GhZAT11-mediated regulation of sugar transport and cell division—thereby filling a key theoretical gap—but also holds dual practical implications. On one hand, controlled wounding treatments can be applied to promote BGT in bulb crops, accelerating germination and shortening the production cycle. On the other hand, it highlights the importance of minimizing mechanical wounding during harvesting, storage, and transportation to prevent untimely sprouting and extend shelf life. The value of science perhaps lies in this duality – it not only tells us how to "awaken", but also reminds us how to "maintain".
Follow the Topic
-
Nature Plants

An online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in