Chimeric antigen receptor T (CART) cell therapy is an innovative form of immunotherapy where human T-cells are engineered with synthetic receptors to target cancer cells. This therapy has shown remarkable success in patients with aggressive blood cancers, and its concept has revolutionized the treatment of cancer. However, despite the impressive overall response rates, the durable response rates remain low1-3. While evaluating the success of CART cell therapy in patients, it was determined that CART cell expansion and persistence are important determinants of response4. A leading cause for limited CART cell persistence in patients is the development of a dysfunctional cellular state, T-cell exhaustion5.
Immunologists have been working to define T-cell exhaustion for years by studying CD8+ T-cells that have been chronically exposed to viral load. From these studies, exhaustion has been defined as a dysfunctional cellular state that is characterized by a reduction in proliferative ability, an increase in the co-expression of multiple inhibitory receptors, and a decrease in the production of effector cytokines such as IL-2, TNF-α, and IFN-γ6. In CART cells, exhaustion has been reported to occur across CART cell constructs and tumor models, but its prevalence varies.
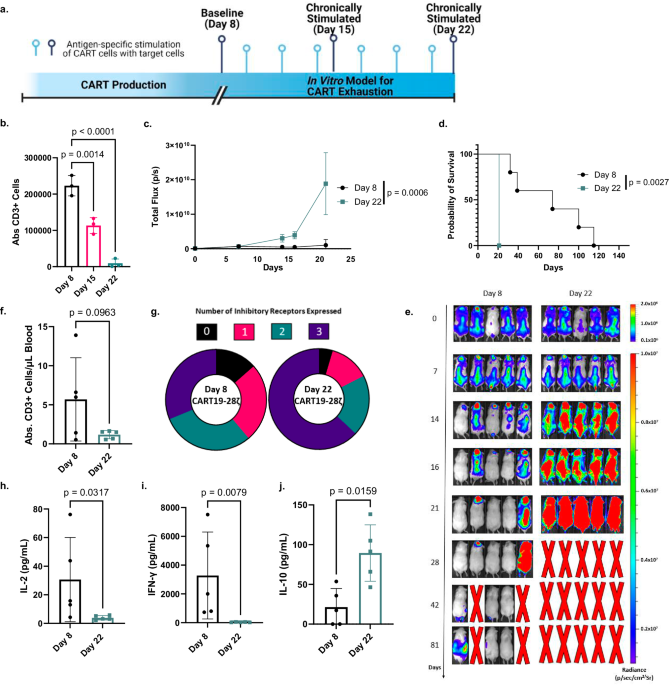
In an attempt to enhance the field’s understanding of CART cell exhaustion and determine strategies to minimize its occurrence, we systematically studied how CART cells become exhausted7. We developed an in vitro model for exhaustion and employed three independent strategies to evaluate the development of CART cell exhaustion. In our in vitro model, we chronically stimulated CART19 cells by adding fresh CD19+ tumor cells every other day for either one week or two weeks. Following chronic stimulation, CART cells showed phenotypic and functional signs of exhaustion such as decreases in expansion, antitumor activity, and effector cytokine production, as well as an increase in the coexpression of inhibitory receptors.
After validating our in vitro model, we applied it in our first approach and completed a genome-wide CRISPR knockout screen in healthy donor CART cells. By comparing gRNA representation in our chronically stimulated CART cells to gRNA representation in our baseline cells, we identified a role for several cytokine signaling pathways, including the IL-4 pathway, in the development of CART cell dysfunction.
For our second approach, we continued to investigate these pathways, along with other regulators of exhaustion, by performing RNA and ATAC sequencing on chronically stimulated and baseline healthy donor CART19 cells from our in vitro model for exhaustion. In this approach, we further validated our in vitro model for exhaustion by verifying that chronically stimulated CART cells develop an epigenetic signature of exhaustion. Then, we performed ingenuity pathway analysis on the list of genes that were both differentially expressed and differentially accessible which revealed IL-4, among other genes, as a top upstream regulator.
Since IL-4 is classically known for its role in polarizing CD4+ T-cells towards a Th2 phenotype, we asked if IL-4 was identified as a top upstream regulator due to the development of exhaustion or if there was an unintended polarization of CD4+ T-cells towards a Th2 phenotype in our in vitro model. To answer this question, we further interrogated phenotypic changes that occurred following chronic stimulation of CART cells. Following chronic stimulation, we saw a sharp decrease in the percent of CD4+ T-cells, and within this declining population there was also a decrease in Th2 polarized T-cells. Further, while we did see an increase in the production of IL-4 following chronic stimulation, it was CD8+ T-cells, not CD4+ T-cells, that were responsible for this increase. Together, this evidence suggests that IL-4 was identified as a top upstream regulator in our in vitro model for exhaustion based on its impact on CD8+ cells rather than its role in Th2 polarization.
Finally, for our third approach, we performed RNA and ATAC sequencing on pre-infusion CART cell products from 6 responders and 6 non-responders in the ZUMA-1 clinical trial that led to the FDA approval of the CART cell product, axicabtagene ciloleucel (axi-cel). From this, we discovered that IL-4 was upregulated in pre-infusion CART cell products from non-responders. Further, when we used our ATAC sequencing data to look specifically at the IL-4 locus, we saw increased chromatin accessibility at the IL-4 locus in pre-infusion products from non-responders in a pattern that mirrored an increase in chromatin accessibility at the IL-4 locus as healthy donor CART cells developed exhaustion in our in vitro model.
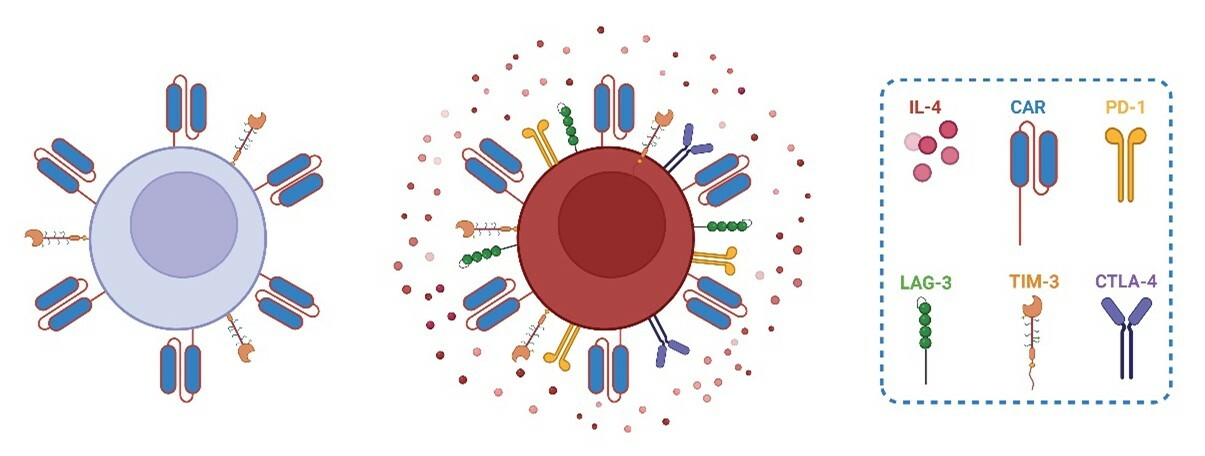
Given that all three of our approaches identified a role for IL-4 in the development of CART cell dysfunction, we then performed in vitro and in vivo experiments to determine how differing IL-4 levels could impact the development of CART cell exhaustion. To start, we added either human recombinant IL-4 (hrIL-4) or diluent control to CART cell cultures as they were being chronically stimulated. The addition of hrIL-4 increased the exhaustion profile of the CART cells as seen by a greater decrease in CART cell expansion a decrease in the production of effector cytokines, and an increase in the coexpression of multiple inhibitory receptors (Fig. 1). This was seen with both bulk CART cells and isolated CD8+ CART cells, indicating that IL-4 can drive exhaustion directly in CD8+ CART cells. Next, we disrupted the IL-4 pathway in CART cells through three methods including 1) CRISPR knockout of IL-4, 2) CRISPR knockout of IL4R, and 3) IL-4 neutralization with a monoclonal antibody. IL-4 pathway disruption through each of these three approaches reduced the incidence of CART cell exhaustion as seen by increases in CART cell expansion, antitumor activity, and effector cytokine production, as well as a decrease in the coexpression of multiple inhibitory receptors.
Figure 1. Summary figure showing a non-exhausted CART cell (left) and a CART cell that has developed exhaustion after being exposed to an abundance of IL-4 (right).
In summary, we identified a novel regulatory role for IL-4 in the development of CART cell exhaustion through a direct effect on CD8+ T-cells. Further, we tested and validated three approaches to improve CART cell activity through IL-4 pathway disruption. Since there are already clinically available monoclonal antibodies that block IL-4’s activity, we believe that this approach may prove to be a translatable strategy to improve durable response to CART cell therapy in the clinic.
References:
1 Neelapu, S. S. et al. 5-Year Follow-Up Supports Curative Potential of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma (ZUMA-1). Blood, doi:10.1182/blood.2022018893 (2023).
2 Locke, F. L. et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol 20, 31-42, doi:10.1016/S1470-2045(18)30864-7 (2019).
3 Schuster, S. J. et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. 380, 45-56, doi:10.1056/NEJMoa1804980 (2018).
4 Wittibschlager, V. et al. CAR T-Cell Persistence Correlates with Improved Outcome in Patients with B-Cell Lymphoma. Int J Mol Sci 24, doi:10.3390/ijms24065688 (2023).
5 Zebley, C. C. et al. CD19-CAR T cells undergo exhaustion DNA methylation programming in patients with acute lymphoblastic leukemia. 37, 110079 (2021).
6 Wherry, E. J. T cell exhaustion. 12, 492-499 (2011).
7 Stewart, C. M. et al. IL-4 drives exhaustion of CD8(+) CART cells. Nat Commun 15, 7921, doi:10.1038/s41467-024-51978-3 (2024).
Follow the Topic
Immunotherapy
Life Sciences > Biological Sciences > Immunology > Immunotherapy
Cytokines and Growth Factors
Life Sciences > Biological Sciences > Immunology > Immune Cell Signalling > Cytokines and Growth Factors
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
A selection of recent articles that highlight issues relevant to the treatment of neurological and psychiatric disorders in women.
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
This Collection aims to bring together research from various domains related to neurodegenerative conditions, encompassing novel insights into disease pathophysiology, diagnostics, therapeutic developments, and care strategies. We welcome the submission of all papers relevant to advances in neurodegenerative disease.
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in