Insights into hyperuricemia amelioration mechanisms of Lactobacillus rhamnosus GG may enable probiotics therapy
Published in Chemistry, Microbiology, and Cell & Molecular Biology
A recent study investigated the mechanism by which Lactobacillus rhamnosus GG (LGG) could have beneficial effects in Hyperuricemia (HUA), demonstrated the potential of a goose model of diet-induced HUA, and LGG is a promising therapeutic dietary supplement for the alleviation of HUA.
About Hyperuricemia (HUA)
Hyperuricemia (HUA) is a metabolic syndrome caused by abnormal purine metabolism, which has become a worldwide metabolic disease with increased prevalence in most countries. The prevalence of HUA is particularly high in coastal and oceanic areas, such as the USA (20%), Japan (25%), and European countries (19%~25%). According to the Chinese guidelines for the diagnosis and treatment of HUA and gout, the number of HUA patients in China increased by 30% between 1998 and 2018, and the number of gout patients increased by 2.18-fold. Among poultry, geese are highly susceptible to gout, and gout in goslings carries high morbidity and mortality rates. Around 500~600 million geese are consumed in China every year, and more than 50% of these geese will suffer gout during the growing period. Although recent studies have noted a relationship between the gut microbiota and gout, whether the microbiota could ameliorate HUA-associated systemic purine metabolism remains unclear. This makes it particularly important to explore the association of the gut microbiota with HUA and therapeutic approaches.
Common Features Between Human Gout and Goose Gout
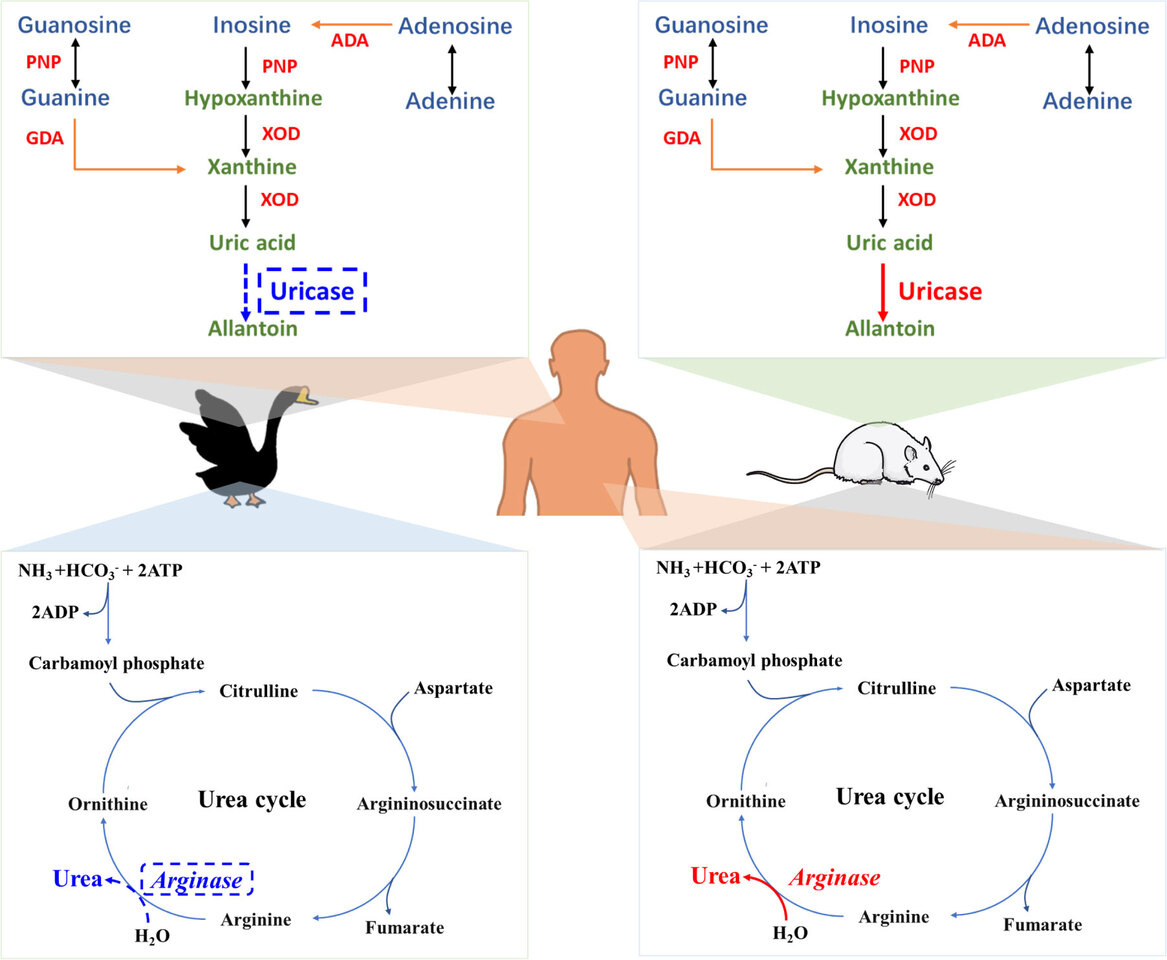
Uric acid is the end product of the metabolism of exogenous purines (derived from food) and endogenous purines (synthesized in the liver, intestines, muscles, kidneys, and vascular endothelium). In other mammals, such as mice, uric acid can be further hydrolyzed into allantoin by uricase, and allantoin is easily excreted from the body. In contrast, humans and avians cannot further hydrolyze uric acid due to a shared inherent defect—uricase deficiency. Moreover, unlike humans, geese are unable to perform the urea cycle due to arginase deficiency, which forces them to excrete ammonia solely through uric acid metabolism. This unique physiological characteristic renders geese more susceptible to gout, making them a valuable animal model for gout research and raising the question of what insights goose gout can provide for the study of human gout.
Progressive Research Exploration
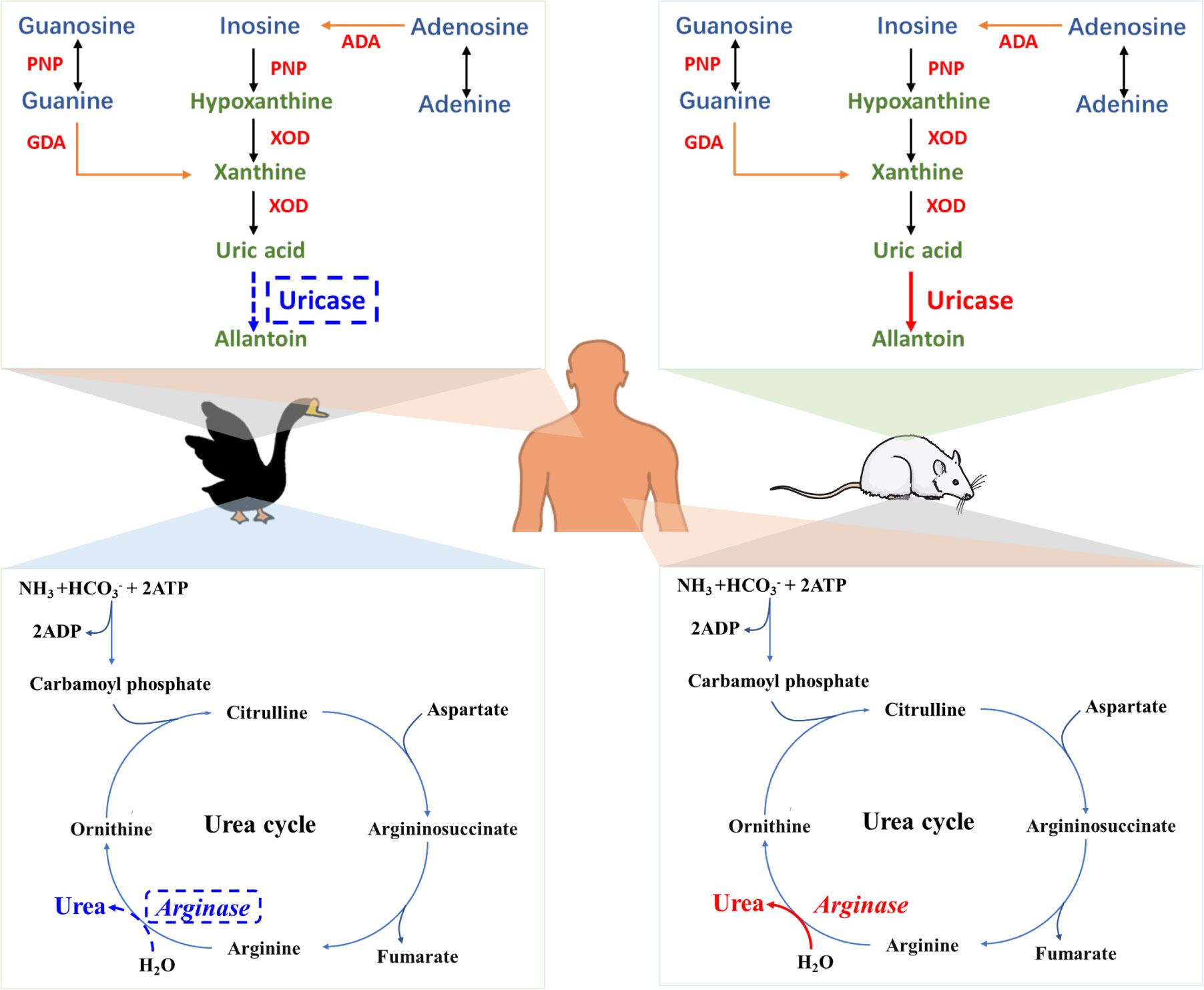
First, we established a goose gout model using high-protein and high-calcium diets, and verified the feasibility of this model by detecting the expression levels of proteins associated with hepatic purine metabolism and renal and intestinal uric acid excretion. Subsequently, through antibiotic clearance, fecal microbiota transplantation assays, and metagenomic analyses, we discovered that Lactobacillus rhamnosus may exert an antagonistic effect on the development of goose gout. Right after this finding, we confirmed via dietary supplementation and gavage experiments that Lactobacillus rhamnosus alleviates goose gout through the gut-liver-kidney axis.
To elucidate the specific mechanism by which Lactobacillus rhamnosus relieves gout, we conducted in vitro experiments and uric acid precursor-nucleoside co-culture assays, combined with whole-genome and metabolomic analyses. The results demonstrated that Lactobacillus rhamnosus can uptake and hydrolyze nucleosides through its endogenous nucleoside transporter proteins, nucleoside hydrolases, and purinergic permeases. Further metabolomic analysis of samples from the full trial revealed that Lactobacillus rhamnosus ameliorates proline depletion caused by gout. Subsequent in vitro cellular assays confirmed that proline alleviates gout by regulating nucleoside and uric acid metabolism in the intestines, liver, and kidneys.
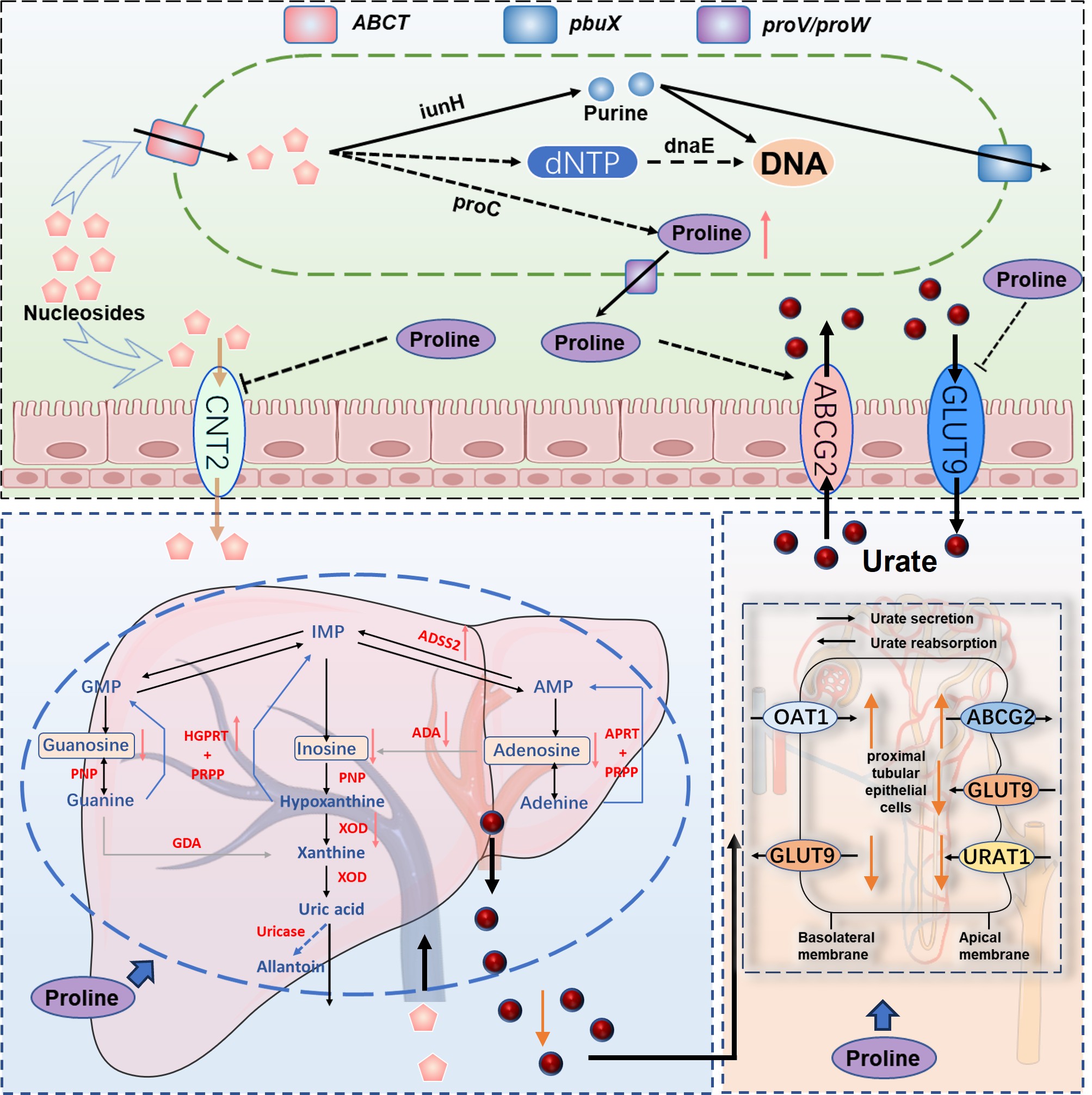
Up to this point, we have relatively comprehensively elucidated the mechanisms underlying the development of goose gout and the gout-alleviating effect of Lactobacillus rhamnosus. However, the gut-liver-kidney axis induction mechanism of proline's action, beyond its direct regulatory effect on uric acid metabolism, remains to be further investigated.
Follow the Topic
Your space to connect: The Myeloid cell function and dysfunction Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine and Cell Biology!
Continue reading announcement


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in