Lipid-based nanodisc vaccines for cancer immunotherapy
Published in Bioengineering & Biotechnology
Lipid complexes can make for excellent delivery vehicles, owing to their biocompatibility and enhanced cellular uptake. Therapeutic proteins and antigens can be incorporated inside lipid complexes, which can be optimized to improve binding, avoid proteolytic degradation, and improve release of the therapeutic payload.
For several years, lipid-based nanostructures have been used as vaccine formulations for delivering relevant antigens and adjuvants that boost the immune response against pathogens or, more recently, tumor cells. In the case of classical vaccination against pathogens, lipid nanoparticles enable the targeted delivery of antigens directly to antigen-presenting cells, which then present those antigens to T cells through the major histocompatibility complex and energize the immune system against the foreign microorganism. In the context of immunizations against tumor cells, neoantigens that result from mutated endogenous proteins can be used to prime the immune system to target transformed cells specifically while avoiding normal tissues that do not express the neoantigen.
In a recent study published in Nature Materials, James Moon and colleagues from the University of Michigan and Bristol-Myers Squibb report a new cancer-vaccine formulation: synthetic high-density lipoprotein (sHDL) nanodiscs loaded with adjuvant CpG oligonucleotides and tumor antigens. The researchers used the vaccine nanodiscs to immunize mice, induce T-cell responses, and inhibit tumor growth. The sHDL nanodiscs, a combination of phospholipids and apolipoprotein-1-mimicking peptides, were shown to be safe to use in human trials in 2003.
To achieve optimal biological activity, the sHDL nanodiscs were functionalized with CpG molecules complexed with cholesterol, and with antigen peptides modified with a cysteine–serine–serine linker (Figure 1). In a series of in vitro studies with bone-marrow-derived dendritic cells (BMDCs), sHDL nanodiscs containing the model antigen peptide OVA257–264 primed the cells more efficiently and led to more sustained antigen presentation than free antigen peptides and soluble CpG. In agreement with these results, OVA257–264 T cells included in the cultures showed evidence of increased activation.
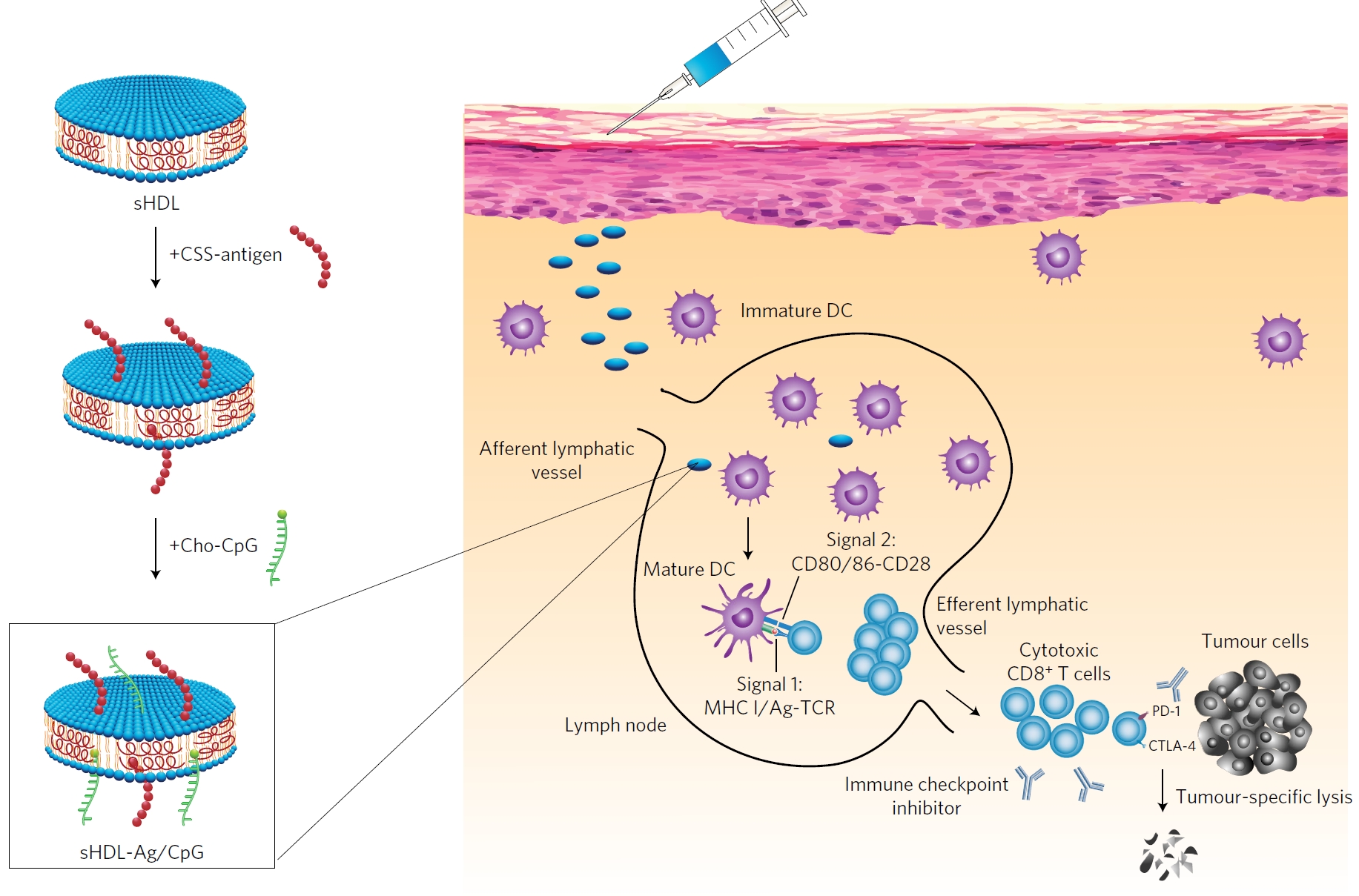
Figure 1: Synthetic high-density lipoprotein (sHDL) nanodiscs, composed of phospholipids and apolipoprotein-1 mimetic peptides, are mixed with cysteine-modified tumour antigen (Ag) peptides and a cholesterol-modified adjuvant (Cho-CpG). This formulation delivers Ag and CpG to antigen-presenting cells (e.g. dendritic cells (DCs)) on draining lymph nodes, boosting tumour Ag-specific CD8α+ cytotoxic T-lymphocyte responses that recognize and kill target cancer cells. The combination of the nanosdiscs with immune checkpoint blockade further amplifies the potency of the vaccination. Reproduced from Figure 1; Kuai, R. et al. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. (2016) doi:10.1038/nmat4822; SpringerNature.
Experiments in mice showed that the nanodiscs accumulated in draining lymph nodes after injection, and that they induced approximately 10-fold higher frequencies of antigen-specific CD8α T cells when compared with free antigen and adjuvant. Furthermore, B16 cancer cells expressing the OVA antigen were unable to establish tumors in vaccinated mice up to 28 days post-challenge, whereas most mice immunized with free antigen and adjuvant died with large tumour masses. These data suggested that the nanodiscs generated a strong and functional immune response against the loaded antigen.
Similar experiments with sHDL nanodiscs bearing tumour neoantigens showed similar efficiency in stimulating the T-cell response, but were not able to induce rejection of tumours expressing the neoantigen in mice. Noting an increase in expression of programmed cell death-1 (PD-1) and of its ligand PD-L1 in the tumour microenvironment following immunization, the authors combined sHDL nanodiscs with immune checkpoint inhibition (anti-PD-1 antibodies). This combination regimen led to tumour regression in ~88% of the mice, and protected survivors against re-challenge up to 70 days after immunization, indicating the establishment of immunological memory.
To immunize mice against a wider range of specificities, sHDLs were also tested in a formulation loaded with multiple antigens and neoantigens expressed by B16F10 melanoma cells. This formulation inhibited tumour growth in vivo without the need for immune checkpoint inhibition (only when multiple antigens were included), confirming the efficiency of this vaccination method. In agreement with the previous experiments, combination of sHDLs bearing multiple antigens with immune checkpoint therapy had the strongest effect, inducing tumour rejection in ~90% of mice. This set of experiments highlights the importance of activating the immune system against a broad range of specificities when seeking to inhibit tumor expansion, and provides further confirmation of the synergies of immune checkpoint inhibition and other cancer therapies.
Highlighted paper:
Kuai, R. et al. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. doi:10.1038/nmat4822 (2016).
Further reading:
Copland, M. J. et al. Lipid based particulate formulations for the delivery of antigen. Immunol. Cell Biol. 83, 97–105 (2005).
Irvine, D. J. et al. Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 12, 978–990 (2013).
Banner credit/source: PhotoDisc/ Getty Images \ Nick Koudis





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in