Multifunctional Dipoles Enabling Enhanced Ionic and Electronic Transport for High‑Energy Batteries
As global demand for sustainable energy surges, the performance ceiling of current battery technologies is increasingly tied to how efficiently ions and electrons move through the cell. Now, a multinational team led by Dr. Yuntong Sun (Nanyang Technological University), Dr. Zhendong Hao (Nanjing Institute of Technology) and Prof. Jong-Min Lee (DGIST) has delivered a panoramic review in Nano-Micro Letters showing how molecular and ionic dipole interactions can push that ceiling higher. The work provides a design playbook for next-generation high-energy batteries that are safer, longer-lasting and wide-temperature-capable.
Why Dipole Interactions Matter
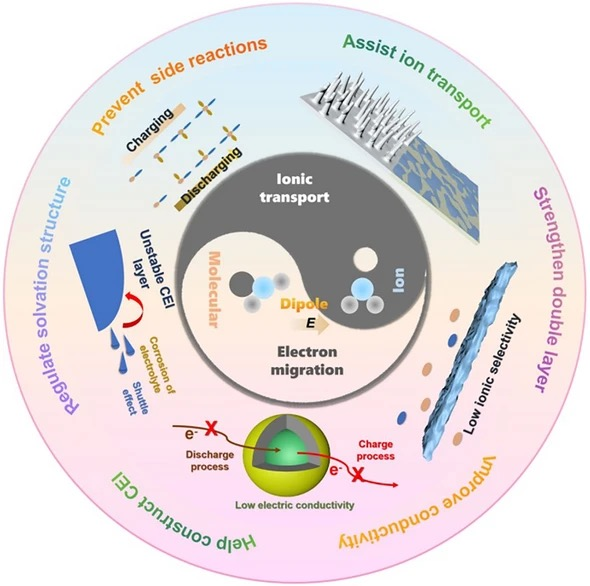
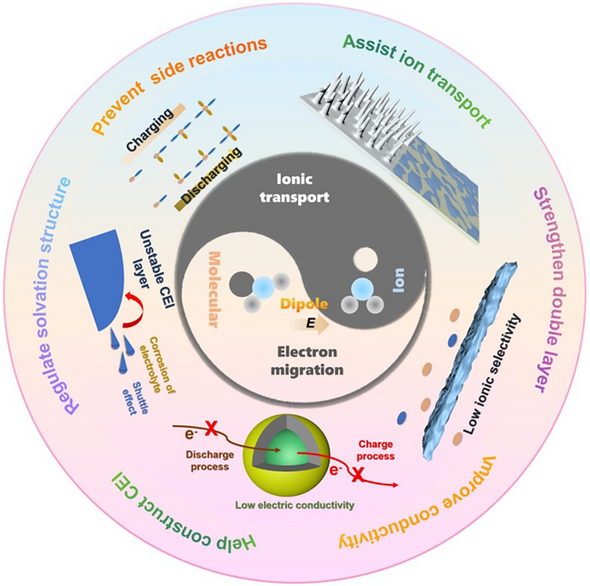
- Energy Density Unlocked: Dipole fields regulate ion-solvent coordination, suppress dendrites, stabilize electrode–electrolyte interfaces and unlock extra capacity from existing cathode chemistries.
- Interface Engineering: Dipoles build robust solid-electrolyte interphase (SEI) and cathode–electrolyte interphase (CEI) layers, cutting parasitic reactions and impedance growth.
- Universal Tool-box: From Li-ion, Li-metal and Li–S to Na-ion and Zn systems, dipole strategies display chemistry-agnostic adaptability across liquid, gel and solid-state formats.
Innovative Design and Features
- Dipole Classifications: Ion–solvent molecule, ion–functional group and additive molecule ion–dipole interactions are dissected with structure–function tables linking specific dipole motifs to performance gains.
- Functional Materials: Crown ethers, ferroelectric BaTiO3, polar carbonates, sulfonamides and nitrile-rich polymers are spotlighted as dipole donors that re-wire solvation sheaths and electric-double-layer topology.
- Array Architectures: Electric-field-assisted vertical alignment, in-situ UV polymerization and asymmetric ceramic/polymer integration create oriented ion highways inside composite electrolytes and separators.
Applications and Future Outlook
- Multi-Level Transport: Dipole-ordered channels raise Li+/Na+/Zn2+ transference numbers (up to 0.82), cut desolvation barriers and enable 5C–10C fast charge without dendrite initiation.
- High-Voltage Stability: Dipole-engineered CEI layers deliver 91 % capacity retention after 100 cycles at 4.3 V (Li||NCM523) and extend oxidative stability of polymer electrolytes to 4.6 V.
- Wide-Temperature Resilience: Strong multiple ion–dipole networks preserve solvation geometry from −60 °C to 100 °C, yielding 89 % capacity retention at 100 °C and 76 % at −40 °C.
- Challenges and Opportunities: The review flags needs for AI-aided dipole design, in-situ characterization databases and scale-up collaboration to translate dipole-boosted coin-cell records into pouch-cell products.
This roadmap underscores the pivotal role of dipole interactions in bridging materials science, electrochemistry and computation for future high-energy storage. Stay tuned for more field-advancing work from Prof. Sun, Prof. Hao and Prof. Lee’s teams!
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in