Optogenetically Controlled “Oncolytic Bacterial Warriors” for Precision Therapy of Solid Tumors
Published in Bioengineering & Biotechnology and Biomedical Research

Cancer is a prominent source of mortality globally, and the worldwide burden is rising1. Live biotherapeutics for sustained delivery of anti-tumor agents have attracted increasing attention in oncology owing to the potential for improved therapeutic efficiency and limited side effects compared to traditional therapies such as chemotherapy and radiation2. Specific bacteria such as Escherichia, Salmonella, Listeria, and Pseudomonas can selectively colonize hypoxic, immunosuppressive, and biochemically unique microenvironments within solid tumors3-5, exhibiting inherent anti-tumor effects based on stimulation of the innate immune system or secretion of natural anti-tumor products6.
For the reduced toxicity to normal tissues triggered by the over-production of therapeutic proteins by engineered bacteria, In 2024, our group developed therapeutically active engineered bacteria mediated by a sono-activatable integrated gene circuit based on the thermosensitive transcriptional repressor TlpA39 (SINGER system)7, This system, with high sensitivity, modularity, and compatibility, allows rapid engineering of multiple payloads in response to US irradiation. However, the need for coupling agents and specialized sonographic equipment increases intervention complexity8, reducing clinical practicality. Moreover, the high temperatures required (42-45 °C) pose risks such as potential thermal damage to healthy tissues8 and unintended activation of endogenous thermo-responsive proteins9.
Light serves as a compelling trigger to tune gene expression in bacteria10. Several blue light-activatable photoswitches have been constructed in bacteria to modulate gene expression based on photosensitive proteins from fungi and bacteria11. However, the limited tissue penetration and potential phototoxicity of blue light hinder the in vivo applicability of blue light–activated photoswitches12.
To overcome these shortcomings, we developed a near-infrared light-mediated PadC-based photoswitch (NETMAP) system based on a chimeric phytochrome-activated diguanylyl cyclase (PadC) and a cyclic diguanylate monophosphate (c-di-GMP)-responsive transcriptional activator MrkH (Fig. 1). Briefly, upon NIR light illumination (710 nm), the chimeric photoreceptor PadC converts intracellular guanosine triphosphate (GTP) into c-di-GMP13, allowing the transcriptional activator MrkH to bind to its chimeric promoter PmrkA and initiate target gene expression.
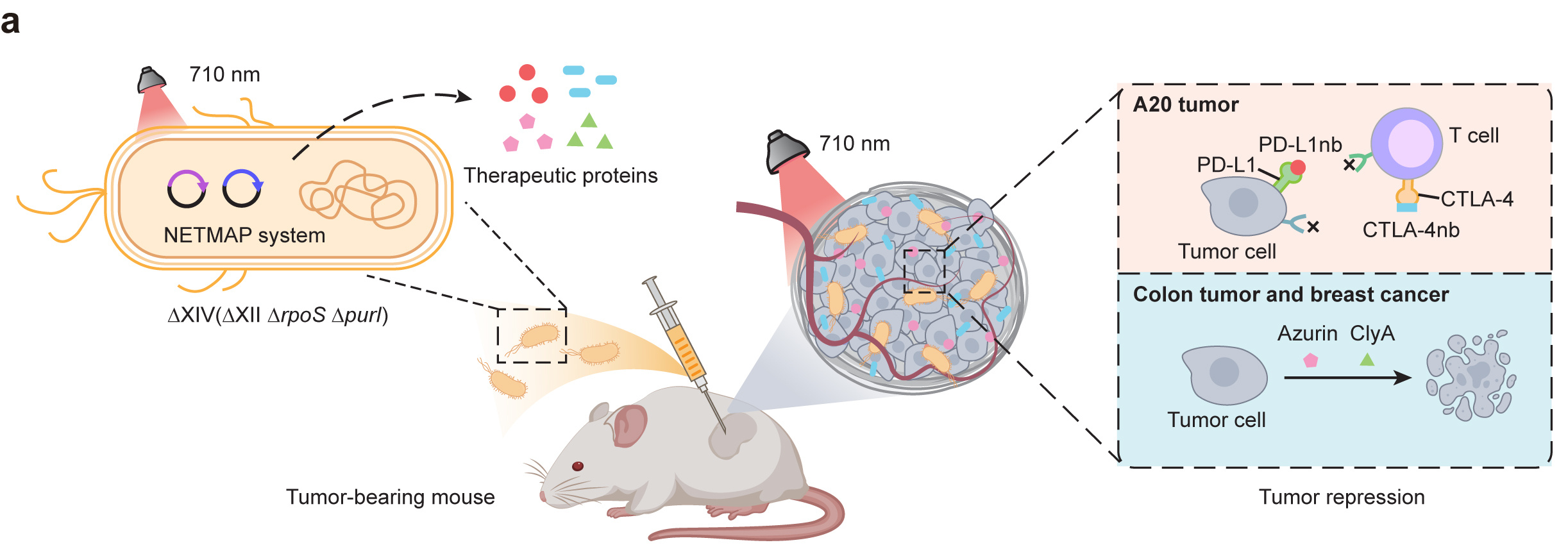
Fig. 1 The NETMAP system-mediated tumor therapy
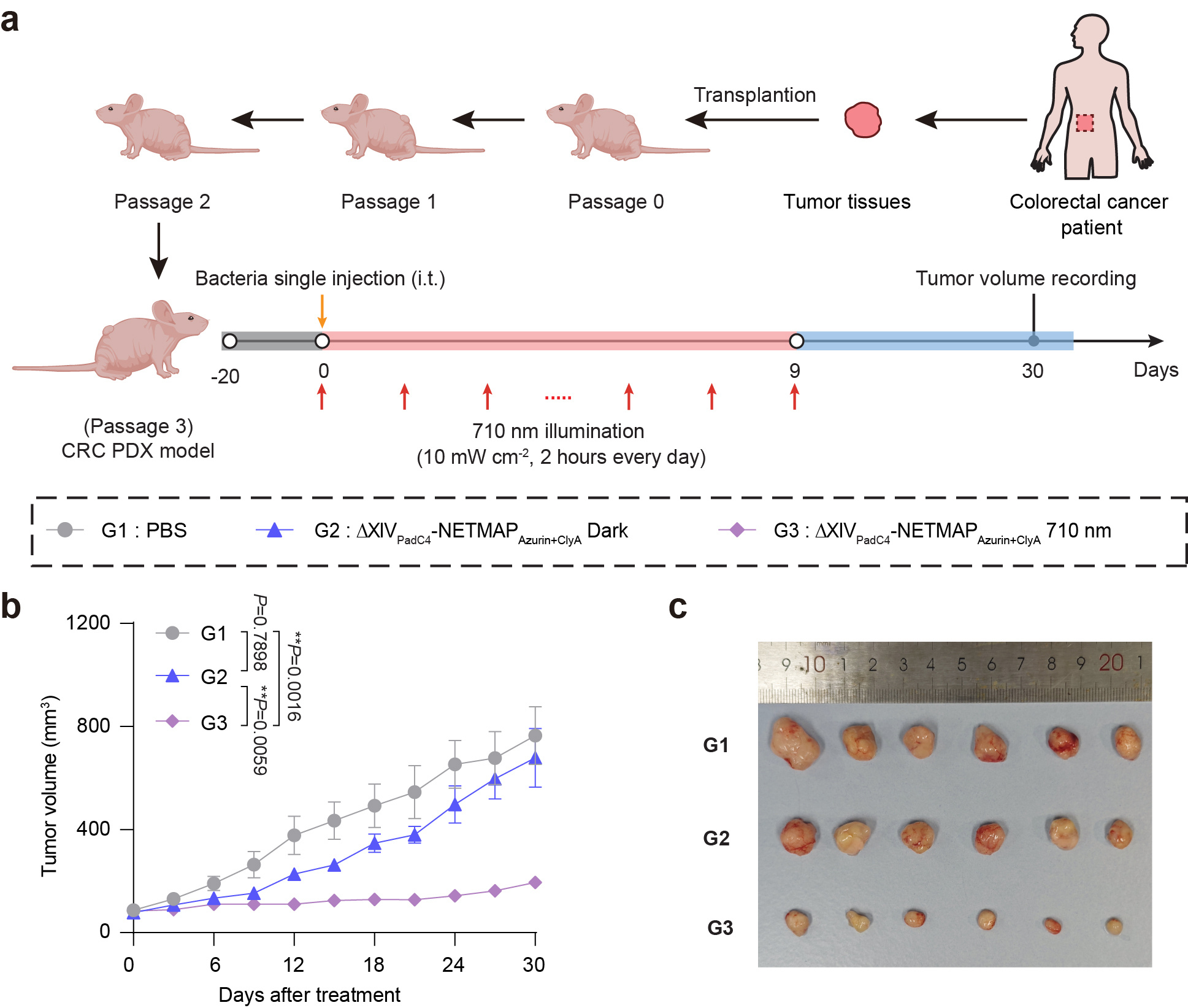
The NETMAP system demonstrated robust activation of reporter gene expression (55-fold) upon NIR light illumination compared to darkness. NIR light-induced production of anti-tumor therapeutic proteins [CTLA-4 nanobody (CTLA-4nb), PD-L1 nanobody (PD-L1nb), Azurin, and Cytolysin A (ClyA)] from ∆XIV significantly inhibited tumor growth and improved the survival rate of mice in various murine tumor models by activating adaptive immunity or inducing apoptosis and lysis of tumor cells. For example, to better simulate clinical patient conditions, we established a patient-derived xenograft (PDX) model using clinically sourced colorectal cancer tissues. Following the injection of engineered oncolytic bacteria, NIR irradiation was applied continuously for nine days, with two hours of exposure per day. Tumor growth was significantly inhibited, and histological analysis further revealed extensive tumor necrosis in the treatment group (Fig. 2).
Fig. 2 NETMAP-mediated tumor therapy in a CRC PDX model
In conclusion, we have built an optogenetic platform integrated into an attenuated S. enteritidis strain, providing a potential strategy to explore combination therapies for various cancers. We anticipate that NIR light-triggered live biotherapeutics should contribute to accurate and effective treatments across multiple diseases by providing customizable outputs and precise dosage control.
Our paper : Qiao, L. et al. Engineered bacteria for NIR light-inducible expression of cancer therapeutics. Nat. Cancer. (2025).
References
1. Wang, H. et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 388, 1459-1544 (2016).
2. Arranja, A.G. et al. Tumor-targeted nanomedicines for cancer theranostics. Pharmacol Res. 115, 87-95 (2017).
3. Zhou, S. et al. Tumour-targeting bacteria engineered to fight cancer. Nat Rev Cancer. 18, 727-743 (2018).
4. Yi, X. et al. Bacteria-triggered tumor-specific thrombosis to enable potent photothermal immunotherapy of cancer. Sci Adv. 6, eaba3546 (2020).
5. Chen, Y. et al. Genetically engineered oncolytic bacteria as drug delivery systems for targeted cancer theranostics. Acta Biomater. 124, 72-87 (2021).
6. Kim, S.H. et al. High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res. 69, 5860-5866 (2009).
7. Gao, T. et al. Sonogenetics-controlled synthetic designer cells for cancer therapy in tumor mouse models. Cell Reports Medicine. 5 (2024).
8. Gao, T. et al. Sonogenetics-controlled synthetic designer cells for cancer therapy in tumor mouse models. Cell Rep Med. 5, 101513 (2024).
9. Lim, J. et al. ASIC1a is required for neuronal activation via low-intensity ultrasound stimulation in mouse brain. eLife. 10, e61660 (2021).
10. Chen, X. et al. An extraordinary stringent and sensitive light-switchable gene expression system for bacterial cells. Cell Res. 26, 854-857 (2016).
11. Ribeiro, I.M.A. et al. Spatial and temporal control of expression with light-gated LOV-LexA. G3 (Bethesda). 12 (2022).
12. Tan, P. et al. Optophysiology: Illuminating cell physiology with optogenetics. Physiol Rev. 102, 1263-1325 (2022).
13. Gourinchas, G. et al. Influence of the N-terminal segment and the PHY-tongue element on light-regulation in bacteriophytochromes. J Biol Chem. 294, 4498-4510 (2019).
Follow the Topic
-
Nature Cancer

This journal aims to provide a unique forum through which the cancer community will learn about the latest, most significant cancer-related advances across the life, physical, applied and social sciences.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in