Overcoming Chemotherapy Resistance in Head and Neck Cancer using PI3K Inhibition
Published in Biomedical Research
The Therapeutic Gap in HNSCC
Head and neck squamous cell carcinoma (HNSCC) remains resistant to the precision-medicine revolution. A decade of genomic research has shown that HNSCC is dominated by loss-of-function alterations in tumor-suppressor genes, rather than readily targetable oncogenic drivers. Only a small fraction of tumors carry HRAS mutations, and immune checkpoint inhibitors produce objective responses in fewer than 20% of patients, with durable remissions in under 10%. Consequently, cisplatin continues to anchor therapy even for advanced disease. Unfortunately, many patients acquire cisplatin resistance, while others display intrinsic resistance from the outset. Our group and others have shown that resistance frequently coincides with hyperactivation of the Nrf2 pathway, which rewires tumor metabolism, dampens chemotherapy and radiation response, and fosters an immunosuppressive tumor microenvironment. Nrf2-driven tumors are notably aggressive, prone to metastasis, and often refractory to standard treatment.
A Window of Opportunity: Targeting the PI3K Pathway
We focused on the PI3K–AKT–mTOR signaling axis, one of the most frequently dysregulated pathways in human cancer. Although approximately 18% of HNSCC tumors harbor PIK3CA mutations, clinical trials of PI3K inhibitors have shown limited benefit—suggesting that mutation status alone does not predict therapeutic response. Two insights shaped our approach. First, NOTCH1-mutant HNSCC tumors are highly sensitive to PI3K inhibition, undergoing apoptosis rather than simple growth arrest—revealing a potential genetic vulnerability. Second, the pan-PI3K/mTOR inhibitor gedatolisib had recently shown potent activity and good tolerability in breast-cancer trials, making it an appealing candidate for repurposing in HNSCC. Our central hypothesis was straightforward: even cisplatin-resistant, Nrf2-hyperactivated tumors remain dependent on PI3K signaling, and pharmacologic blockade of this pathway could restore treatment sensitivity.
Inside the Study: Multimodal Experiments
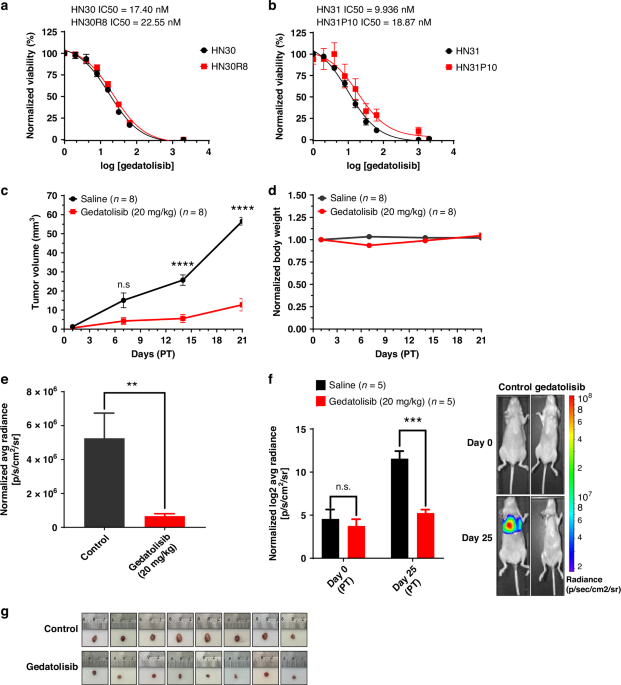
We employed clinically relevant orthotopic models with confirmed Nrf2 activation exhibiting local invasion, metastatic spread, and humanized immune compartments.
- In vitro, resistant cell lines were exposed to gedatolisib to assess effects on proliferation, cell-cycle arrest, gene expression, and metabolism.
- In vivo, mice bearing established tumors received gedatolisib, with evaluation of tumor growth, survival, and tumor–immune dynamics.
- Complementary transcriptomic, metabolomic, and spatial analyses provided a comprehensive view of molecular and cellular effects.
Key Findings
- PI3K as a central driver: The pathway was consistently hyperactive in resistant tumors, and genetic screens identified PI3K signaling as a dominant vulnerability across multiple genomic backgrounds.
- Potent anti-proliferative effect: Gedatolisib induced G₂/M arrest, halting proliferation in both HPV-positive and HPV-negative HNSCC lines.
- Restoration of cisplatin sensitivity: PI3K inhibition synergized with cisplatin, re-sensitizing resistant cells via induction of autophagy and senescence, which enhanced cytotoxicity.
- Metabolic disruption: Gedatolisib impaired fatty-acid metabolism, negating the Nrf2-mediated metabolic advantage of resistant cells.
- In vivo efficacy: Treatment markedly reduced tumor growth and metastasis while prolonging survival in mouse models.
- Microenvironment remodeling: Gedatolisib decreased tumor hypoxia and reduced regulatory T-cell infiltration, creating a milieu more permissive for immune activation.
- Favorable tolerability: Unlike earlier PI3K inhibitors, gedatolisib was well-tolerated in vivo, consistent with its established clinical safety record.
Follow the Topic
-
British Journal of Cancer

This journal is devoted to publishing cutting edge discovery, translational and clinical cancer research across the broad spectrum of oncology.
Latest Content
Why is Singapore Identified in Global Research as Number One? How Physical Activity and Education Excellence Created a Global Leader




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in