Parental experiences orchestrate locust egg hatching synchrony by regulating nuclear export of precursor miRNA
Published in Ecology & Evolution, Cell & Molecular Biology, and Zoology & Veterinary Science

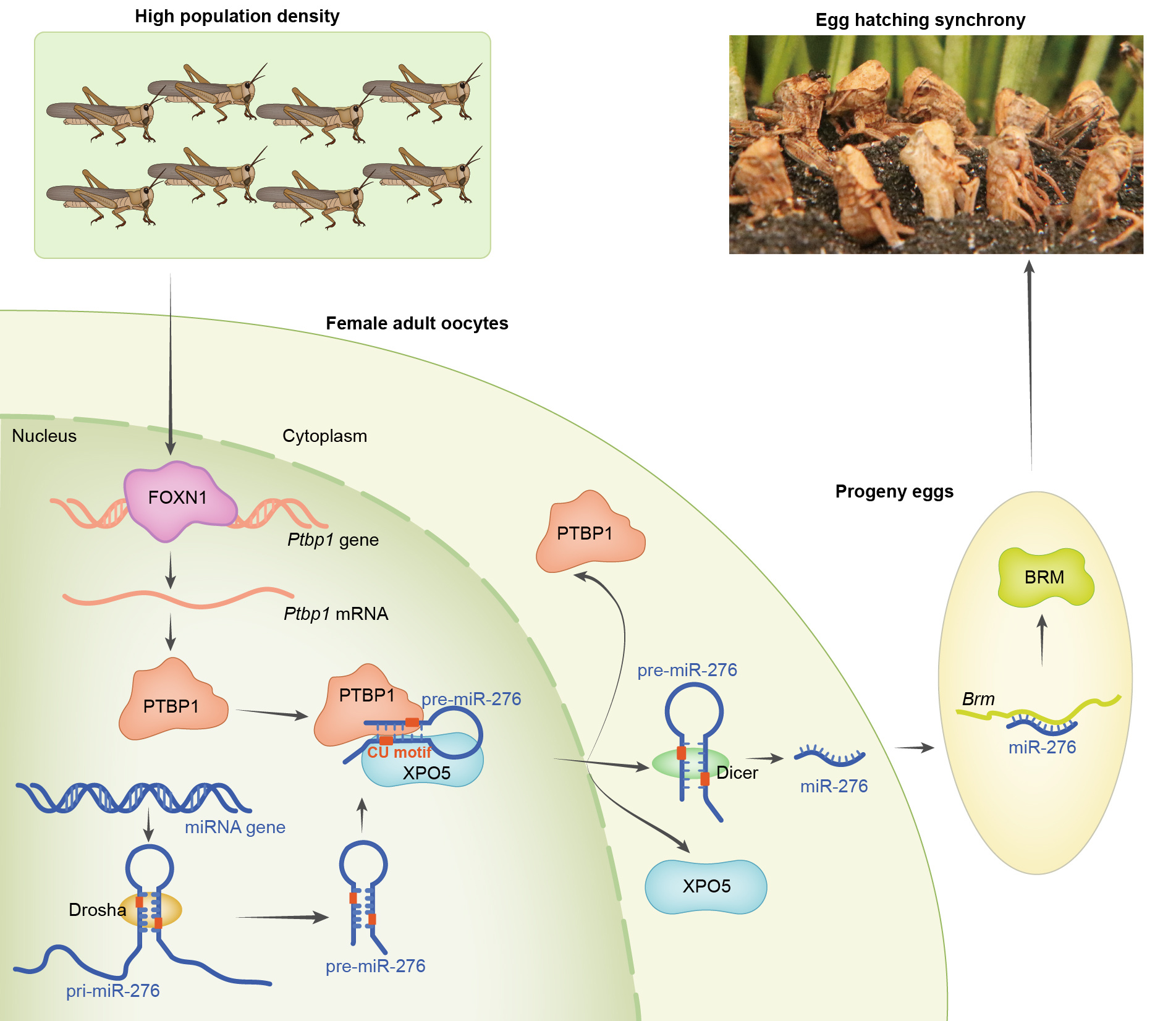
Our story begins with the biological synchrony of locusts. Synchrony and asynchrony represent two distinct strategies employed by animals. In some species, such as altricial birds, hatching asynchrony of eggs is a prevalent phenomenon. Although high mortality rate caused by this strategy seem disadvantageous, it is likely that such asynchrony aids the offspring in adapting to limited resource availability [1]. However, for group-living animals, synchronous development strategy is particularly significant. Among certain species of mammals and birds, synchronous reproduction can enhance reproductive success [2, 3]. Developmental synchrony is crucial for beetles and sea turtles to evade the threat of predators [4]. The locust applies various reproductive strategies in response to population density changes. Low density induces asynchronous development of female ovaries and progeny eggs, whereas high density results in synchronous development of female ovaries and progeny eggs [5, 6]. The synchronized development paves the way for the aggregation and long-distance migration of locusts.
In 2016, our group revealed that of egg-hatching synchrony in locusts is controlled by high density experiences of female locusts. High density induces the expression of a microRNA, miR-276, which activates the target gene Brahma (Brm) through abolishing the secondary structure of Brm mRNA in the ovaries [5]. Brm is an essential gene for embryo development and nucleosome stability [7, 8]. Thus, the elevated expression of miR-276 and BRM ensures the stability of embryonic development, ultimately leading to the egg-hatching synchrony of gregarious locusts [5]. Although the downstream pathway of miR-276 is elucidated by our previous study, the specific mechanism that triggers the increased expression of miR-276 in the ovaries in response to high density remains unclear. More importantly, the transduction pathway that connects environmental signals to reproductive small RNAs is one of the major unresolved questions in the field of transgenerational effects.
To elucidate the mechanism underlying how high density regulates the expression of miR-276, we delved into the biogenesis steps of miR-276 specifically in the terminal oocytes which is directly related to progeny eggs. In addition, we utilized HEK 293T cells that lack endogenous miR-276 to study the regulatory mechanism involved in miR-276 biogenesis. By detecting and analyzing the intermediate products of miR-276, we found that the high expression of miR-276 in the terminal oocytes of gregarious locusts was attributed to the elevated nuclear export of precursor miR-276 (pre-miR-276).
Which is the regulator for the nuclear export of pre-miR-276? RNA pull-down is an ideal technique to probe the proteins that interact with specific RNAs. Additionally, we performed RNA interference (RNAi) in female locusts and the functional assay in HEK 293T cells to screen for the potential proteins involved in the nuclear export of pre-miR-276. Through this comprehensive approach, we identified an RNA binding protein, Polypyrimidine tract-binding protein 1 (PTBP1), as a regulator in nuclear export of pre-miR-276 in locusts.
As an RNA binding protein, PTBP1 usually acts downstream of signal transduction pathway. How does PTBP1 respond to high density? We found that a transcription factor (TF), Forkhead box protein N1 (FOXN1), was induced in the terminal oocytes by high density signal. Activation of Ptbp1 by FOXN1 induced the high expression of miR-276 in the terminal oocytes of gregarious locusts.
How does PTBP1 regulate the nuclear export of pre-miR-276? The locust PTBP1 was a nucleus-cytoplasm shuttling protein, however, we found that it cannot function independently of the miRNA exportin protein Exportin 5 (XPO5). Instead, PTBP1 facilitated the nuclear export of miR-276 by interacting with XPO5. Interestingly, the function of PTBP1 was not universal in promoting the nuclear export of all pre-miRNAs. Unlike XPO5, PTBP1 cannot recognize the stem-loop structure of pre-miRNAs. By conducting a detailed sequence analysis of pre-miR-276 and performing mutational assays in HEK 293T cells, we discovered that PTBP1 exerted the function of exportin by specifically recognizing the “CU motifs” present in sequence of pre-miR-276. This recognition allows PTBP1 to target pre-miR-276 for nuclear export in a specific and precise manner, rather than affecting global miRNA expression levels. In addition, we found the conservation of “CU motifs” in the sequences of insect pre-miR-276, and we validated the mechanism of PTBP1-facilitated nuclear export in Drosophila.
As we know, PTBP1 is a canonical protein which exert crucial roles in neuronal development. The unexpected discovery of PTBP1's function in reproductive system of locusts attracted our attentions. We wanted to explore whether PTBP1-promoted nuclear export of pre-miR-276 in female terminal oocytes mediates the hatching-synchrony of progeny eggs. To investigate this, we knocked down PTBP1 expression in female locusts and performed a rescue assay by overexpressing miR-276 after PTBP1 knockdown. Our results confirmed that PTBP1 indeed mediates egg-hatching synchrony by promoting the expression of miR-276.
Taken together, high density activates the FOXN1-PTBP1/XPO5-miR-276 pathway in oocytes of female locusts to regulate the hatching synchrony of progeny eggs. This mechanism is crucial for enabling future swarming and migration behaviors of locusts. Our study revealed the key initial step in environment-induced transgenerational effects. We identified PTBP1 as a new partner of XPO5, facilitating the nuclear export of pre-miRNA by specifically recognizing the “CU motifs”. Due to the prevalence of “CU motifs” in the locust genome, we will further investigate the role of PTBP1 in the nuclear export of other RNAs beyond pre-miR-276. Moreover, we will delve into the transduction pathway that links the neuron system to reproductive system.
References:
- Stenning MJ. Hatching asynchrony, brood reduction and other rapidly reproducing hypotheses. Trends Ecol Evol 1996; 11: 243-6.
- Emlen ST, Demong NJ. Adaptive Significance of Synchronized Breeding in a Colonial Bird: A New Hypothesis. Science 1975; 188: 1029-31.
- Matsumoto-Oda A, Ihara Y. Estrous asynchrony causes low birth rates in wild female chimpanzees. Am J Primatol 2011; 73: 180-8.
- Spencer R-J, Thompson MB, Banks PB. Hatch or wait? A dilemma in reptilian incubation. Oikos 2001; 93: 401-6.
- He J, Chen Q, Wei Y et al. MicroRNA-276 promotes egg-hatching synchrony by up-regulating brm in locusts. Proc Natl Acad Sci USA 2016; 113: 584-9.
- Chen D, Hou L, Wei J et al. Aggregation pheromone 4-vinylanisole promotes the synchrony of sexual maturation in female locusts. Elife 2022; 11.
- Tamkun JW, Deuring R, Scott MP et al. brahma: A regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2SWI2. Cell 1992; 68: 561-72.
- Shi J, Zheng M, Ye Y et al. Drosophila Brahma complex remodels nucleosome organizations in multiple aspects. Nucleic Acids Res 2014; 42: 9730-9.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in