Regenerative medicine at the intersection of materials science and biology
Published in Bioengineering & Biotechnology
Of the collection of articles that I’ve intermittently written, and carefully stored on this repository (since December 2016), the underlying themes are interdisciplinarity and translational research, with a common purpose to advance biomedical engineering. This theme is exemplified in biomaterials-assisted regenerative medicine strategies with interdisciplinary applications in cardiology, renal medicine, bone tissue engineering, immune and neural engineering, broadly classified under the umbrella term of tissue engineering, with applications across a bench to clinic pipeline.
Regenerative medicine and device biocompatibility
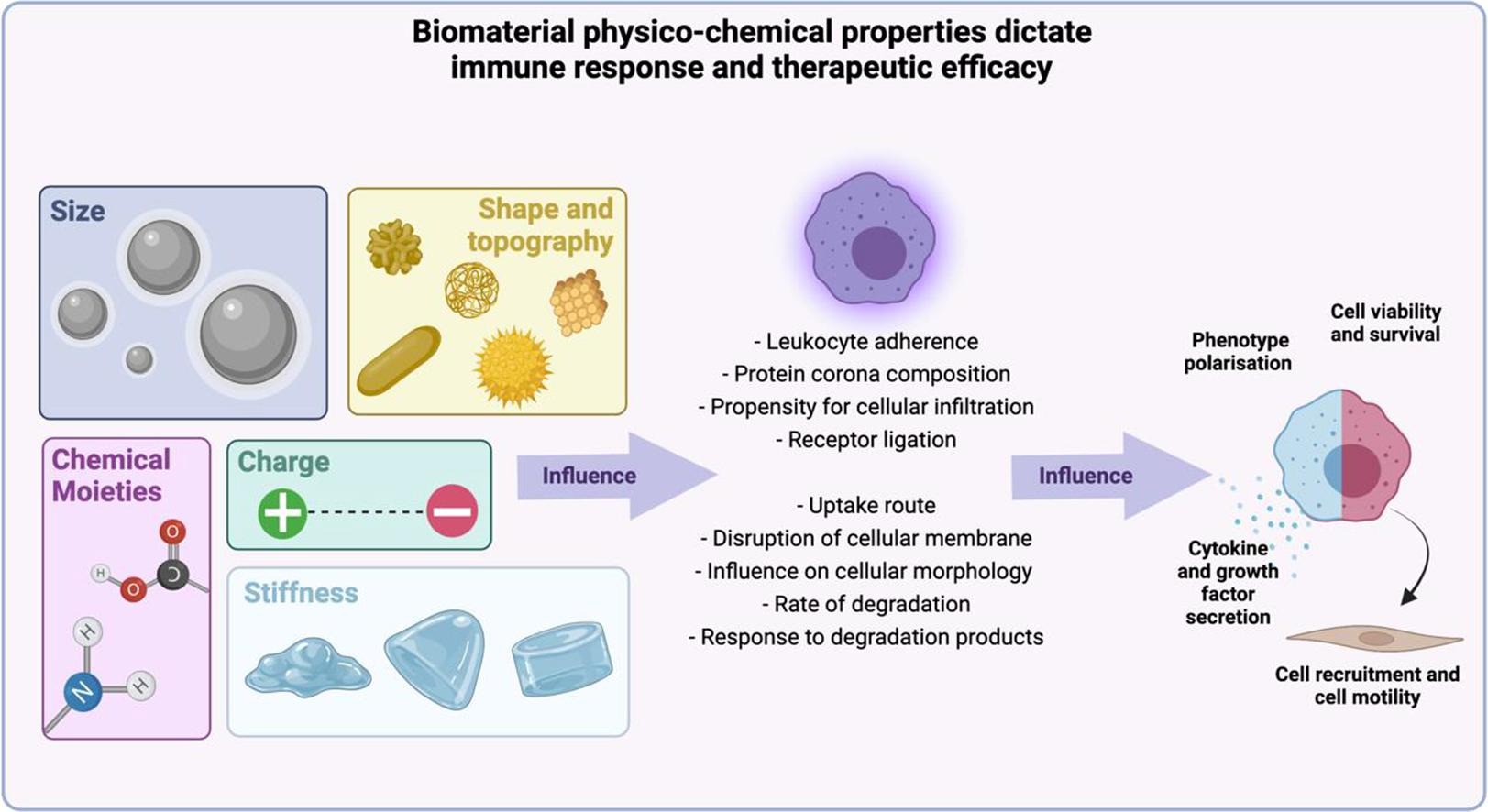
The biocompatibility of biomaterials depends on tissue-material interactions at the site of implantation that initially rely on favorable interactions between macrophage-biomaterial implantable surfaces - that are then optimized for immunomodulation and eventual clinical translation [Bozinovski 2021]. Biomaterials are developed in accordance with ISO standards to determine their physicochemical characteristics and cytotoxicity, to ensure successful outcomes in clinical trials. These include assessing the foreign body reaction, inflammation, encapsulation, and the accumulation of macrophages in the peri-implant zone (Figure 1).
In my previous articles I have communicated about cell-biomaterial interactions in cardiology, the development of immuno-materials for immune-engineering, bioinspired microfabrication for tissue engineering, and four-dimensional biofunctionalization to bioengineer biologically inspired artificial tissues for applications in regenerative medicine. This post offers a quick recap on the advancements and primary methods of biomaterials engineering for regenerative repair across the fields of cardiology, bone tissue engineering, renal medicine, and the disciplines of neural and immune engineering.
Regulating the pathophysiological environment as a therapeutic strategy – precision medicine.
Ideally, implantable biomaterials must be able to regulate inflammation, vascularization, and tissue modeling at the cell material interface. Macrophage-endothelial cell niches are crucial to regulate the biocompatibility of materials, and this complexity can be tailored to restore homeostasis in various diseases as a new therapeutic strategy of precision medicine [Guan 2023]. Precision medicine is based on the development of disease-specific molecular classification methods that accurately reflect clinical behavior [Yin 2023].
Although interactions between macrophages and endothelial cells during disease progression are widely known, much yet remains to be known of the extracellular matrix and its role in the intercellular process relative to cell-biomaterial interactions [Guan 2023],[Boghdady 2021]. Cell-generated forces including tensegrity are foundational across several biological and pathological processes [Ingber 2003], and cell-biomaterial interactions themselves are subject to tensegrity, during biomaterial-assisted cell proliferation and growth to use them as extracellular matrices. Tenable properties allow the study of macrophage-endothelial cell fate, and regulate innate and adaptive immunity to facilitate angiogenesis, and regeneration [Guan 2023].
Hydrogels can be developed with tunable stress relaxation properties to regulate stem cell fate and activity in cell culture, independently of the material’s initial elastic modulus, degradation, and cell adhesion density [Chaudhuri 2015]. Bioengineers have exemplified this niche with bone tissue engineering, where cell spreading, proliferation, and osteogenic differentiation of mesenchymal stem cells can be improved for the gels to undergo faster relaxation (Figure 2).
Mesenchymal stem cells can also form a mineralized, collagen-I-rich matrix, much like bone, by interacting with rapidly relaxing hydrogels. The in vivo relaxation is mediated by cell- and extracellular matrix interactions to promote biomaterials design for cell culture. Mechanical stress too can regulate cell function by activating or tuning signal transduction pathways, where mechanotransduction converts mechanical stimuli primarily to a chemical response, another aspect to consider when designing biomaterials that respond to mechanical cues [Alenghat 2002].
Intelligent materials to heal living tissue.
In general, smart materials include a sensor to detect an input signal, an actuator as a responsive component for adaptive function, and actuators with four types of integral materials [Nabila 2022], including:
- Shape memory alloys, i.e., metals that can revert to their original shapes when the temperature is changed.
- Piezoelectric ceramics – constructs that expand and contract in response to an applied electric field, while generating an electrical field where the dimensions are altered.
- Magnetostrictive materials – analogous to the piezoelectric constructs, and responsive to changes in a magnetic field and able to alter the magnetic or electric field [Rios 2023], and
- Four-dimensional bioprinting – to incorporate the additional dimension of time within the printed 3-D scaffolds, with an external stimulus exposed to the printed construct to change its shape or functionality to self-assemble and self-heal in time after implantation [Faber 2023].
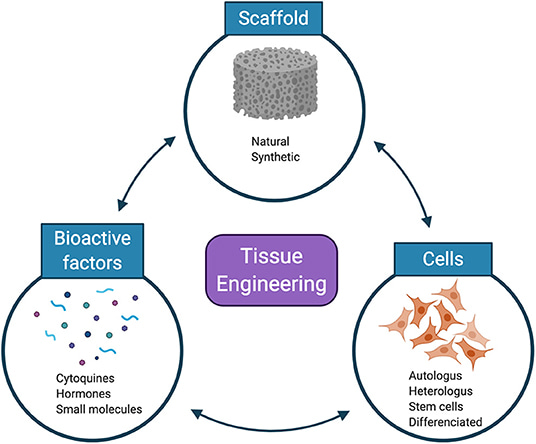
Biomaterials in the form of hydrogels, carriers, and scaffolds play a significant role to anchor cells and generate functional tissues [Nguyen 2019]. Material compositions typically include polyglycolic acid (PGA), poly-l-lactic acid (PLA), poly-DL-glycolate (PLGA), polyvinyl alcohol and their derivatives (Figure 3).
Cardiac tissue engineering
A multitude of emerging, interdisciplinary techniques have lent themselves to engineer state-of-the-art fabrication methods for basic research and clinical applications in cardiac tissue engineering [Nguyen 2019]. These include 3-D scaffolds to support cardiomyocyte growth and maturation, including efforts for stem-cell based therapy via gene-editing to repair gene mutations, and reveal the genetic molecular clock. Cardiomyocytes rely on cardiac cell alignment to maintain tissue microarchitecture and biological functions. Such methods include a combination of topographical patterning, and chemical treatment. In 1997, bioengineer Thomas Eschenhagen pioneered the development of engineered heart tissue by combining cardiomyocytes and non-cardiomyocytes within an extracellular matrix to form a cardiac patch for drug screening, disease modeling, and cardiac regeneration [Eschenhagen 1997].
As with all biomaterial-cell interactions, cardiac cells require biomimetic environmental conditions to support early growth conditions to differentiate and bind onto a scaffold [Majid 2020]. Since the etiology of cardiac disease is also based on genetic-related factors, gene-editing methods too can intervene at the molecular level to regulate the alignment, adherence, and differentiation of cells during cardiac tissue engineering [Nguyen 2019]. Such biomaterials are highly biocompatible and include native constructs such as fibrinogen, collagen, alginate, and silk as natural sources of robust biocompatibility, when combined with cells to improve cardiac function post myocardial infarction [Nguyen 2019]. Engineered heart tissues are structural constituents for drug screening, disease modeling, and cardiac regeneration to replace myocytes post myocardial infarction, and are suited for clinical translation [Weinberger 2017].
Neural tissue engineering
Natural polymers have excellent cell adhesion and growth properties and are therefore capable of promoting cell-biomaterial interactions, cell adhesion, and extracellular matrix deposition suited for neural tissue engineering [Doblado 2021]. Collagen is a fundamental native constituent that can be combined with proteins and polymers for neural tissue engineering, and is an approved biopolymer for clinical use [Kehoe 2012]. Several biomaterials are of interest for neural tissue engineering, including gelatin nanoparticles, and hyaluronic acid that supports neurite outgrowth to increase the survival rates for therapeutic applications in the peripheral nervous system and the central nervous system.
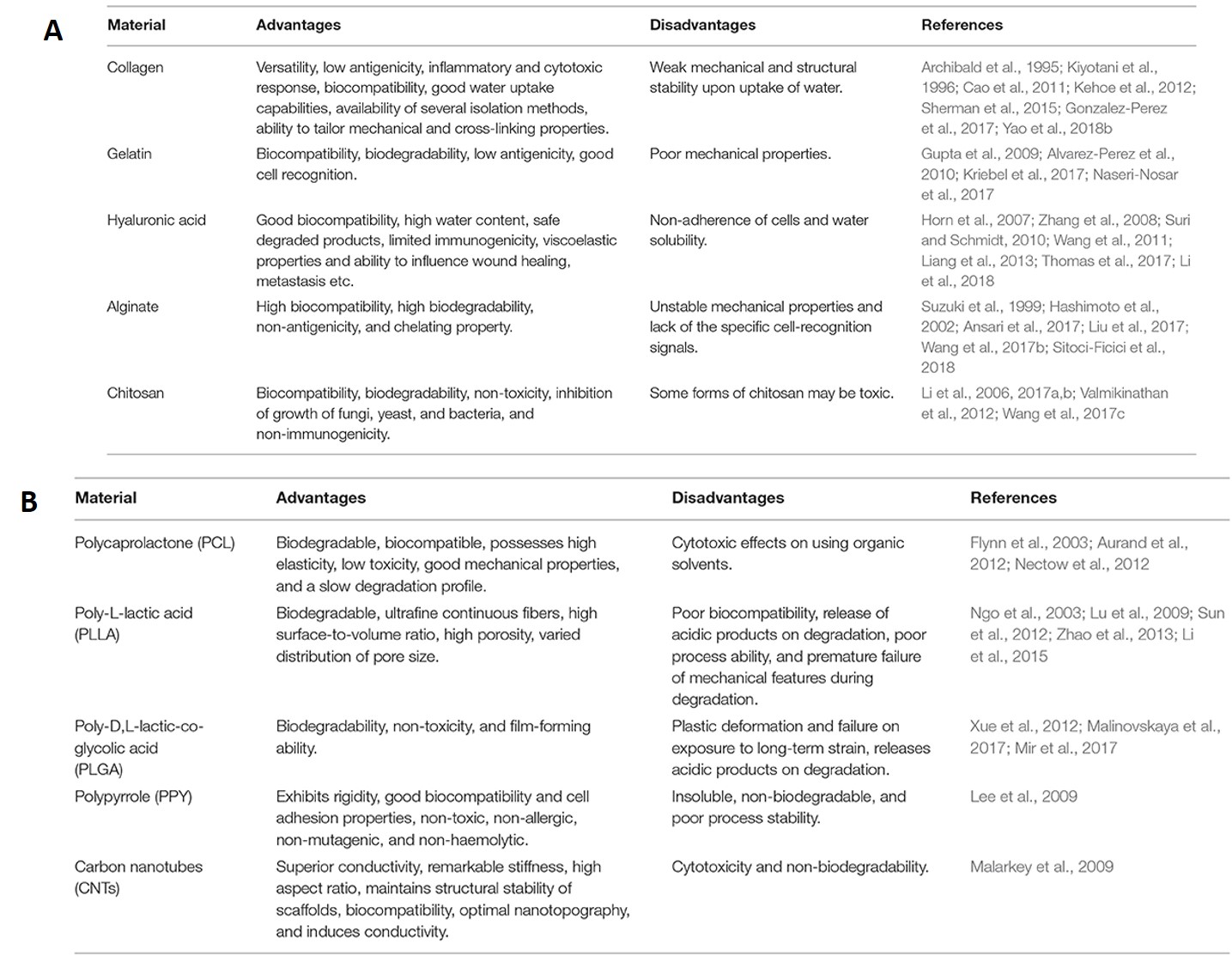
As with all scaffolds, biomaterials for neural tissue engineering must be biodegradable and biocompatible without producing an inflammatory response [Doblado 2021]. The materials should maintain porosity to exchange oxygen, nutrients, and factors between nerve guidance conduits – to form superior conductive biomaterials for electrical guidance during neural tissue regeneration [Bhangra 2016]. Neurons have a complex anatomy, the biomaterial-guided process of regeneration or repair must accompany significant scientific advances relative to cell-based studies, nanotechnologies, and advanced biomaterials, to better understand the nervous system in the lab, for precision medicine applications [Anderson 2008]. A list of native and synthetic biomaterials suited for neural tissue engineering are listed on table 1.
Bone tissue engineering
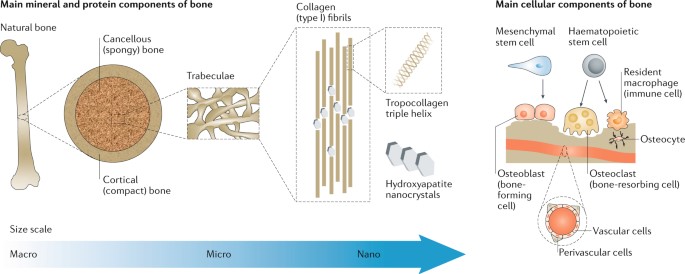
Materials underlying bone regeneration and reconstruction are another niche in biomaterials engineering that rely on the combination of biodegradability and biocompatibility. Bone tissues are naturally regenerative, however, bone defects that exceed the critical size threshold of more than 2 cm require assisted healing [Annamalai 2019]. The gold standard treatments for large bone defects can be performed annually, although the associated risks in ageing individuals have led to the advent of the field of bone tissue engineering [Koons 2020].
This niche, is however, not new; the first reports go back to the nineteenth century, when surgeons investigated the use of calcium phosphate as bone grafts [Dorozkhin 2013] (Figure 5). Implants have since evolved with advances in materials synthesis and processing, alongside an improved understanding of bone biology and structure [Koons 2020]. The field of bone tissue engineering research can design materials with synthetic and native constructs to outperform the existing gold standard of autografts and allografts [Bhumiratana 2016].
Synthetic polymers offer greater possibilities for chemical modifications and molecular alterations when compared to natural polymers, which include poly-(ε-)-caprolactone – although it lacks features to promote cell adhesion, alongside poly-L-lactic acid, poly (ethylene glycol), and bioactive glasses – a bioceramics constituent. The mechanical performance of metals relative to their Young’s moduli and tissue interaction are suited for biomaterials integration, and include biomaterials made of titanium, magnesium, and graphene, to impart both bioactivity and promote bone calcification [Koons 2020].
Renal tissue engineering for renal medicine:
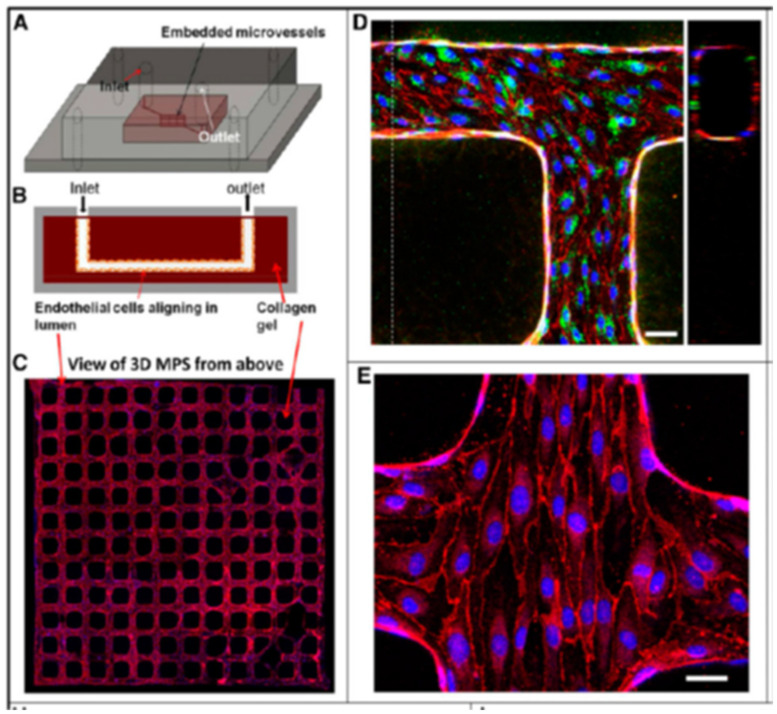
This section will also include a quick note about renal tissue engineering to enhance kidney vasculature. While the technique is presently incorporated to engineer individual renal structures or small organoids, further studies in vascularization with organoids in microfluidic systems can pave the way for large-scale, whole tissue-engineered kidneys through a top-down bioengineering approach, suited for kidney transplantation, based on translational research [Lebedenko 2021]. Key tissue engineering methods in the field include repopulating decellularized kidney scaffolds with autologous cells as a promising method for whole-kidney tissue engineering.
The development of stem-cell derived miniature organoids offer a different approach to bottom-up engineering that starts with individual stem cells that differentiate to a renal lineage, to form kidney structures through self-assembly. This approach holds incredible potential to bioengineer immunocompatible kidneys with translational value by using a patient’s autologous cells [Dzobo 2018]. Organ chip models (that I've often written about) are further suited as finely tuned cellular microenvironments for a wide range of applications across drug screening to assess pharmacoepidemiologic interactions, for toxicological studies, and disease modeling [Lebedenko 2021].
Immune engineering
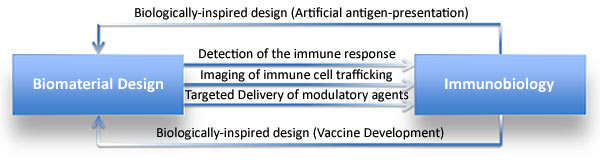
To wrap things up, I revisit immune materials engineering from the archives, another innovative biomaterial devised to create biologically inspired materials for immune intervention (Figure 7). Immunomaterials form an active research focus area to generate bioengineered constructs to impart adaptive immunity [Luksza 2017]. Recent advances of the field are centered on creating engineering tools and principles to investigate and regulate the immune system, with applications across multiple cases of immune diseases. The field is significant in sustained drug delivery to transfer a desired biomolecule to a specific cell population in time. Advances of the concept are suited to develop immunoengineered vaccines with diverse materials to protect against malaria, to act as a nicotine suppressant, and for cancer immunotherapy [Luksza 2017].
Outlook - Translational science from the bench to the clinic
This post is an amalgamation of existing methods in biomedical engineering suited across multiple disciplines that combine materials science with bioengineering. Advances in the collective fields include the capacity to incorporate microfluidics, regenerative medicine, and advanced biomaterials to repair tissues at the molecular and cellular level, suited for tissue reconstruction and patient-aided healing processes with a broader outlook on precision medicine and translational science. The concept builds on pre-existing strategies while introducing advanced methods to recreate tissue constructs from a bench to clinic approach.
Header Image: Tissue Engineering and Regenerative Medicine. Trinity Center for Biomedical Engineering
References
- Bozinovski T et al. Macrophages, the main marker in biocompatibility evaluation of new hydrogels after subcutaneous implantation in rats, Sage journals, doi: https://doi.org/10.1177/08853282211046119
- Guan Y. et al. Engineering biomaterials to tailor the microenvironment for macrophage–endothelium interactions, Nature Review Materials, doi: https://doi.org/10.1038/s41578-023-00591-9
- Yin F. et al. DNA-framework-based multidimensional molecular classifiers for cancer diagnosis, Nature Nanotechnology, doi: https://www.nature.com/articles/s41565-023-01348-9
- Boghdady C. et al. Revisiting tissue tensegrity: Biomaterial-based approaches to measure forces across length scales, APL bioengineering, doi: 10.1063/5.0046093
- Ingber D. et al. Tensegrity I. Cell structure and hierarchical systems biology, Journal of Cell Science, doi: 10.1242/jcs.00359
- Chaudhuri O. et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity, Nature Materials, doi: https://doi.org/10.1038/nmat4489
- Alenghat F. et al. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins, Signal transduction knowledge environment, doi:10.1126/stke.2002.119.pe6
- Nabila et al. Encyclopedia of Smart Materials, Elsevier, doi: 978-0-12-815733-6
- Rios B. et al. Mechanically programming anisotropy in engineered muscle with actuating extracellular matrices, Cell, doi: https://doi.org/10.1016/j.device.2023.100097
- Faber L. et al. Translational biomaterials of four-dimensional bioprinting for tissue regeneration, Biofabrication, doi:https://iopscience.iop.org/article/10.1088/1758-5090/acfdd0
- Ngyuen A. et al. Cardiac tissue engineering: state-of-the-art methods and outlook, Journal of Biomedical Engineering, doi: https://doi.org/10.1186/s13036-019-0185-0
- Eschenhagen T. et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system, doi: 10.1096/fasebj.11.8.9240969
- Majid Q. et al. Natural Biomaterials for Cardiac Tissue Engineering: A Highly Biocompatible Solution, Frontiers, doi: https://doi.org/10.3389/fcvm.2020.554597
- Weinberger F. et al. Engineering Cardiac Muscle Tissue, Circulation Research, doi: https://doi.org/10.1161/CIRCRESAHA.117.310738
- Doblado L. et al. Biomaterials for Neural Tissue Engineering, doi: https://www.frontiersin.org/articles/10.3389/fnano.2021.643507/full
- Kehoe S. et al. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy, doi: 10.1016/j.injury.2010.12.030
- Bhangra K. et al. Using Stem Cells to Grow Artificial Tissue for Peripheral Nerve Repair, Stem Cells International, doi: 10.1155/2016/7502178
- Anderson J. et al. Foreign body reaction to biomaterials, Seminars in Immunology, https://doi.org/10.1016/j.smim.2007.11.004
- Annamalai Injectable osteogenic microtissues containing mesenchymal stromal cells conformally fill and repair critical-size defects, doi: https://doi.org/10.1016/j.biomaterials.2019.04.001
- Koons G. et al. Materials design for bone-tissue engineering, Nature Reviews Materials, doi: https://doi.org/10.1038/s41578-020-0204-2
- Dorozkhin S. V. et al. A detailed history of calcium orthophosphates from 1770s till 1950, Materials Science and Engineering: C, doi: https://doi.org/10.1016/j.msec.2013.04.002
- Bhumiratana S et al. Tissue-engineered autologous grafts for facial bone reconstruction, Science Translational Medicine, doi: 10.1126/scitranslmed.aad5904
- Lebedenko C. et al. Enhancing Kidney Vasculature in Tissue Engineering—Current Trends and Approaches: A Review, doi: 10.3390/biomimetics6020040
- Dzobo K. et al. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine, doi: 10.1155/2018/2495848
- Luksza M. et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy, doi: https://doi.org/10.1038/nature24473





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in