
Cancer is a leading cause of death word-wide with lung cancer being one of the most deadly and most varied category (1). To understand the mechanism of cancer metastasis better, we focused on Epithelial-Mesenchymal Transition (EMT); more specifically, the cytoskeletal rearrangement that occurs during EMT (2). The up and down regulation of a library of proteins during EMT has been studied previously and are commonly used as markers for tracking EMT (3), but relatively less attention has been dedicated to understanding the cytoskeletal aspect of EMT. Though protein levels are an accurate marker of EMT, the individual proteins and their expression levels often vary drastically from one cell line to another. The cytoskeletal reorganization (stress fiber formation), on the other hand is relatively ubiquitous across different cell types. Also, the enhanced motility and invasiveness of mesenchymal (metastatic) cells are integrally interconnected to their cytoskeletons, making it a good candidate for tracking EMT in different cells.
Multiple reports have hypothesized and identified intermediate phenotypic states in the EMT process (4) and in this work (https://www.nature.com/articles/s42003-022-03358-0), we have identified the unique cytoskeletal feature of such an intermediate phenotype in lung cancer cells. This phenotype is a true intermediate in terms of protein expression and mechanical properties as well. We have shown that, during EMT, randomly oriented stress fibers first form in these cells through a Rho/ROCK pathway mediated mechanism. Then these fibers are aligned parallelly to one another through a cross-talk between the Wnt and ERK/MEK genetic cascades.
The term “cytoskeletal reorganization” is often used qualitatively to distinguish between extreme differences in their organization. But for a gradual process like EMT, a more quantitative tool is required to identify the subtle changes as well as intermediate states. In our work, we developed an image quantification tool named Statistical Parametrization of Cell Cytoskeleton (SPOCC) which can quantify the amount of stress fibers in these cells along with their positions and relative orientations from simple fluorescence microscopy images. SPOCC uses a curvelet transform based approach to separate the filaments/fibers from the artefacts and background noise. The filaments are then fit to straight line segments (with a given length) to extract a binary image of the fibers with their length, orientation and position. To quantify their relative alignment, Orientational Order Parameter (OOP) is calculated from the list of angles, which is a measure of how parallel the individual fibers are.
Using SPOCC, we demonstrated that OOP is an accurate reporter for EMT in both single cells and populations. In fact, OOP is more reliable marker of EMT than other, more traditional features such as fluorescence intensity or aspect ratio of cells. SPOCC has also been shown to be a mid-throughput nondestructive tool for drug screening. Along with improved throughput, SPOCC can track faster biological processes, typically occurring at a timescale of minutes to 12 hours, which would be impossible to study using traditional biophysical techniques such as motility and invasion assays. The reason for the enhanced time-resolution of SPOCC is because SPOCC in limited only by the time it takes to capture a fluorescent image. Though SPOCC cannot boast the levels of throughput of RNA sequencing or other high throughput assays, SPOCC is a non-destructive technique which makes it a valuable tool in cases where sample is precious. More importantly, SPOCC can quantify any cytoskeletal rearrangements with sub-cellular resolution, which can be extremely relevant for cell biological studies. Future studies can build a correlation library between cytoskeletal features and other physical and genetic properties of the cell so that the amount of destructive or low throughput experiments can be reduced.
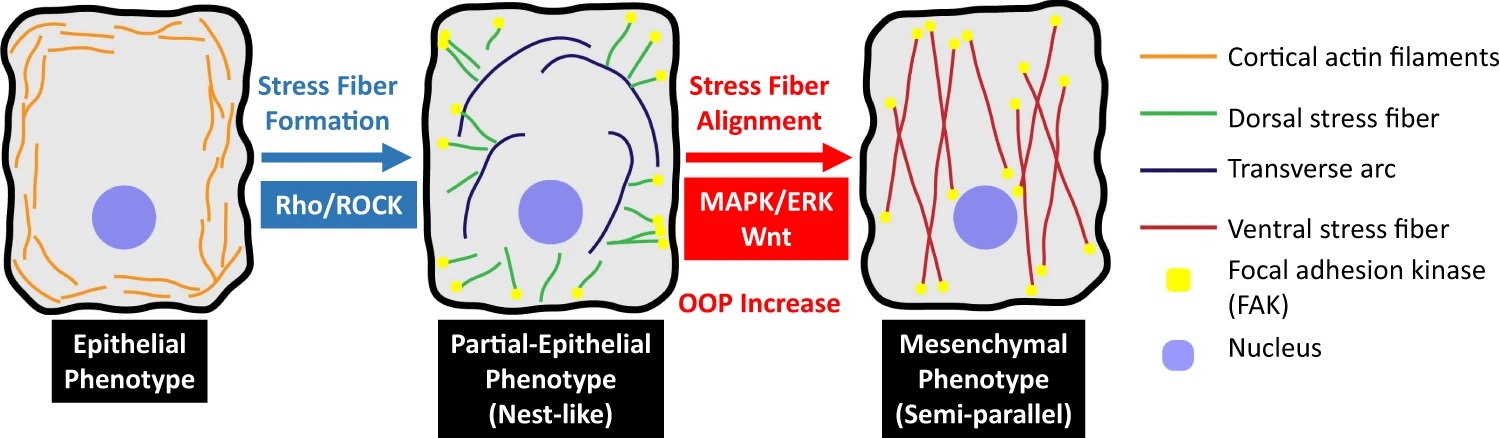
Figure 1: Schematic of cytoskeletal reorganization and the genetic protocols involved in Epithelial-Mesenchymal Transition.
In conclusion, we have shown that formation and alignment of stress fibers during EMT are two distinct processes, controlled by different parts of the EMT genetic program. We have developed a non-destructive image quantification tool, SPOCC, that can reliably track EMT and potentially other biological processes. This technique is extremely suited for the niche of fast biological processes that rearrange the cell cytoskeleton, providing sub-cellular information and direct or indirect measurement of physical properties of cells.
References:
- Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA. Cancer J. Clin. 70, 7–30 (2020).
- Haynes, J., Srivastava, J., Madson, N., Wittmann, T. & Barber, D. L. Dynamic actin remodeling during epithelial-mesenchymal transition depends on increased moesin expression. Mol. Biol. Cell 22, 4750–4764 (2011).
- Ko, J., Winslow, M. M. & Sage, J. Mechanisms of small cell lung cancer metastasis. EMBO Mol. Med. 13, (2021).
- Karacosta, L. G. et al. Mapping lung cancer epithelial-mesenchymal transition states and trajectories with single-cell resolution. Nat. Commun. 10, 1–15 (2019).
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in