Storming the Citadel: Delivering Antibodies into Cancer Cells
Published in Chemistry

Antibodies as Drugs
The 1984 Nobel Prize in Physiology or Medicine was awarded to Jerne, Köhler and Milstein for the derivation of monoclonal antibodies [1]. Some 17 years later, with the approval of the first antibody-based cancer treatment, rituximab, the floodgates opened. By 2021 approximately 40 anticancer antibody therapeutics had been approved in the USA and EU, with several more under review [2]. The clinical impact of these drugs has been transformational: a few important examples are trastuzumab and pertuzumab (breast cancer), pembrolizumab (melanoma, lung cancer), ipilimumab (melanoma, renal cancer), naxitamab (neuroblastoma) and the antibody-drug conjugate, enhertu (breast cancer) [3-5]. The game-changing efficacy of antibody drugs is reflected in their commercial value; it is estimated that the worldwide market for monoclonal antibodies for cancer will reach close to $90 billion by 2024 [6].
Antibody therapeutics then represent some of the most powerful weapons in the fight against cancer, but they are limited in a crucial way – they cannot enter cells; the anti-cancer properties of current agents depend on their interaction with cell surface molecules. This is a significant drawback because the majority of oncogenic drivers reside inside the cell, where they orchestrate uncontrolled cellular proliferation. While small molecule inhibitors can reach the intracellular milieu they are unable to interrupt all pertinent signaling pathways, especially those involving proteins that lack suitable binding pockets. Delivery of antibodies into the intra-cytoplasmic and intra-nuclear spaces of cancer cells would allow these proteins, the root cause of cancer, to be drugged.
The Delivery Challenge
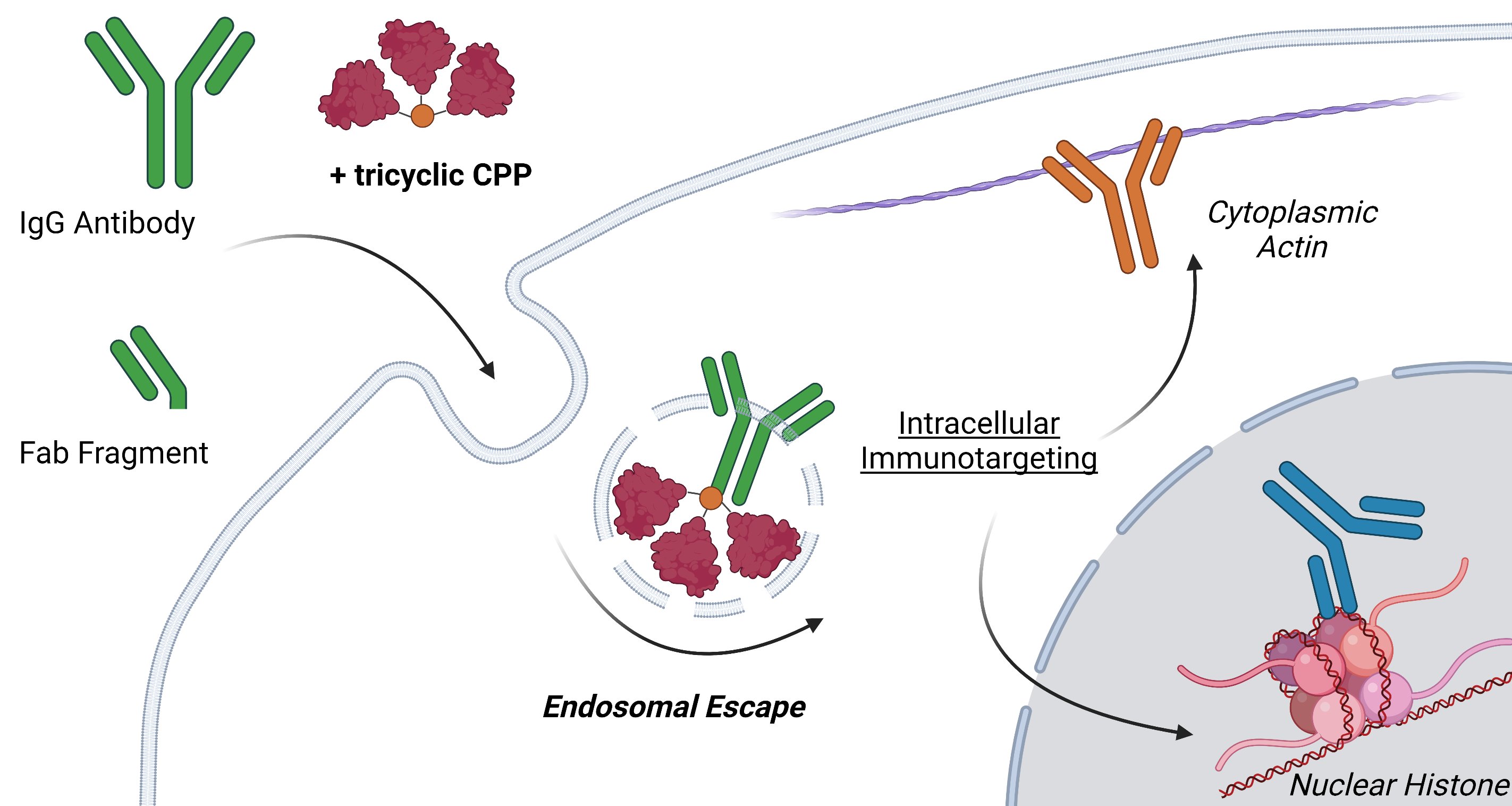
To be effective, antibody delivery systems must be potent at low, clinically-achievable, concentrations and non-toxic so that healthy cells are unharmed. To address this challenge we designed a cell-penetrating construct that reflects two important concepts: first, the clustering of peptides in multimers markedly increases their potency and, second, cyclization of individual peptides results in cell entry of the antibody “cargo” by endocytosis, leaving the plasma membrane unperturbed.
Synthesis of Tricyclic Cell-Penetrating Peptides
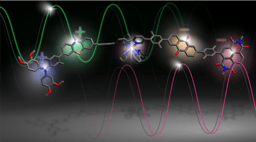
A lead trimeric peptide compound, tri-cTat B, is based on a tetrakis scaffold featuring alkyne functional groups (introduced by copper catalysed azide–alkyne cycloaddition) and azido-functionalized cyclized Tat peptides. Tat, derived from the human immunodeficiency virus 1 (HIV1), has cell-penetrating and nuclear-localizing properties. For initial evaluation, we attached a fluorophore to the fourth site of the tetrakis core giving a fluorescent, barrel-like structure, in which the three Tat-cycles form coiled helices arranged in a compact, off-parallel fashion.
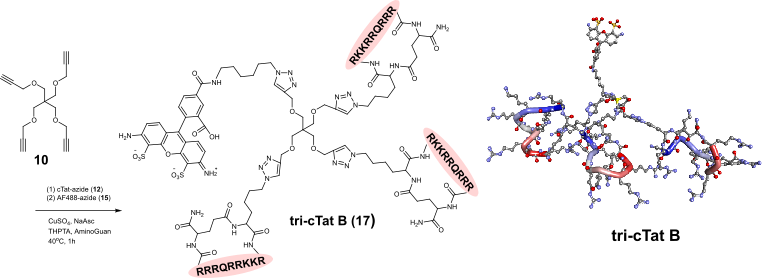
Figure 1. Synthesis of tri-cTat B from tetramer-alkyne starting material and azido-cyclic Tat peptide. In silico-generated ball-and-stick models of tri-cTat B optimized using a Dreiding-like forcefield.
Potency, Uptake and Tolerability
In live cancer cell microscopy experiments we observed successful uptake of tri-cTat B, even at low concentration (1 μM), followed by rapid endosomal release after 10 minutes. In contrast, there was no internalization of monomeric Tat even when added at ten times the concentration. Interestingly, a variant, tri-Tat A, with a similar structure but formed from linear Tat peptides, interacted with the plasma membrane through pore formation. Importantly, this difference in uptake mechanism rendered tri-cTat B less toxic than its linear counterpart.
Internalised Antibodies Retain Their Function
With a potent, non-toxic cell-penetrating agent in hand we investigated the ability of tri-cTat B to deliver antibodies and antibody fragments into cancer cells. Whole IgG and antibody Fab fragments were taken up into human cancer (HeLa) cells incubated with tri-cTat B. Importantly, the concentration of cargo antibodies used in these experiments was within the range that is achievable in vivo (typical dosing of therapeutic antibodies results in plasma concentrations of about 2 µM). Importantly, we showed that antibodies retain the ability to bind their target epitope following tri-cTat B-mediated cell entry. For example, an antibody directed against the intranuclear protein histone H2B was delivered into HeLa cells and, using a combination of immunofluorescence microscopy and proximity ligation assays, we showed association of the internalised antibody with H2B in the histone octamer.
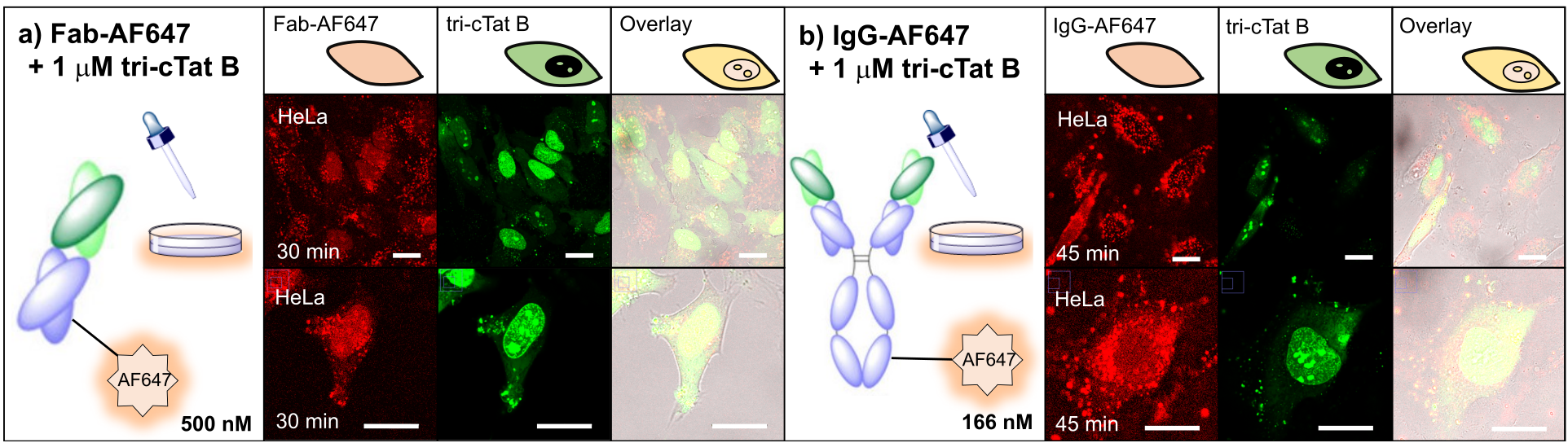
Figure 3. Co-delivery of antibodies and antibody fragments in live HeLa cells using tri-cTat B. Images show a homogeneous distribution of Antibody / Fab in the cytosol and nucleus of cells with green nucleoli staining typical of tri-cTat B delivery.
Outlook
In summary, we report a highly potent, non-toxic intracellular delivery agent, capable of transporting functional antibodies into cancer cells. This work emerged from our interest in developing radioimmunotherapeutics that precisely target cells harboring specific cancer-associated mutations and simultaneously deliver short range electron-emitting radioisotopes for theranostic applications [7, 8]. We envisage that the discovery of tri-cTat B and variants will aid efforts to bring these agents to clinical trial. Our overall aim is to use tri-cTat B for the development of intracellular antibodies to treat previously undruggable targets in cancer.
For the full report please see: https://www.nature.com/articles/s41557-021-00866-0
References
[1] Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495-7.
[2] The Antibody Society. Therapeutic monoclonal antibodies approved or in review in the EU or US. (date accessed 10th Feb. 2022); www.antibodysociety.org.
[3] Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol. 2021;18:327-44.
[4] Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab Deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610-21.
[5] Zahavi D, Weiner L. Monoclonal antibodies in cancer therapy. Antibodies (Basel). 2020;9.
[6] https://www.nature.com/articles/d43747-020-00765-2. Moving up with the monoclonals. Biopharma Dealmakers,2019.
[7] Cornelissen B, Able S, Kartsonaki C, Kersemans V, Allen PD, Cavallo F, et al. Imaging DNA damage allows detection of preneoplasia in the BALB-neuT model of breast cancer. J Nucl Med. 2014;55:2026-31.
[8] Bavelaar BM, Lee BQ, Gill MR, Falzone N, Vallis KA. Subcellular Targeting of Theranostic Radionuclides. Front Pharmacol. 2018;9:996.
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in