Achieving double charge accumulation via sequential excitation
Published in Chemistry
Artificial photosynthesis offers a promising path to CO2-neutral fuels or transportable green energy.1 To produce such fuels, substrate molecules must be reduced more than once in energetically uphill, energy-storing reactions. However, because photons are delivered in discrete quanta, each photon can drive only a single one-electron reduction. Among other challenges, this limitation means that direct two-electron reductions are not photochemically feasible. To circumvent the formation of unstable radical intermediates, a promising approach is to develop catalysts capable of accumulating two electrons before transferring them to the substrate in a single, concerted reaction.
Competing with Photoinduced Charge Recombination
Single electron transfers in molecules are well studied using Donor–Photosensitizer–Acceptor (D–P–A) assemblies, which temporarily separate an electron from the resulting hole. These studies have been extremely valuable for understanding the basics of electron transfers.2 However, when these assemblies were re-excited by a second photon, the desired accumulation of charges (two electrons or two holes) always failed.3 The reason is rather simple: Once charges are separated, the redox landscape is effectively inverted, turning the stored electron on the acceptor into a strong electron donor and conversely, the stored hole on the donor into a strong electron acceptor. Re-excitation therefore leads to recombination rather than charge accumulation. Researchers have achieved temporary multiple charge storage using architectures containing two photosensitizers,4 but the excitation of both chromophores within a useful amount of time requires photon intensities many orders of magnitude higher than sunlight.
Tailor-made molecule for efficient long-distance charge storage
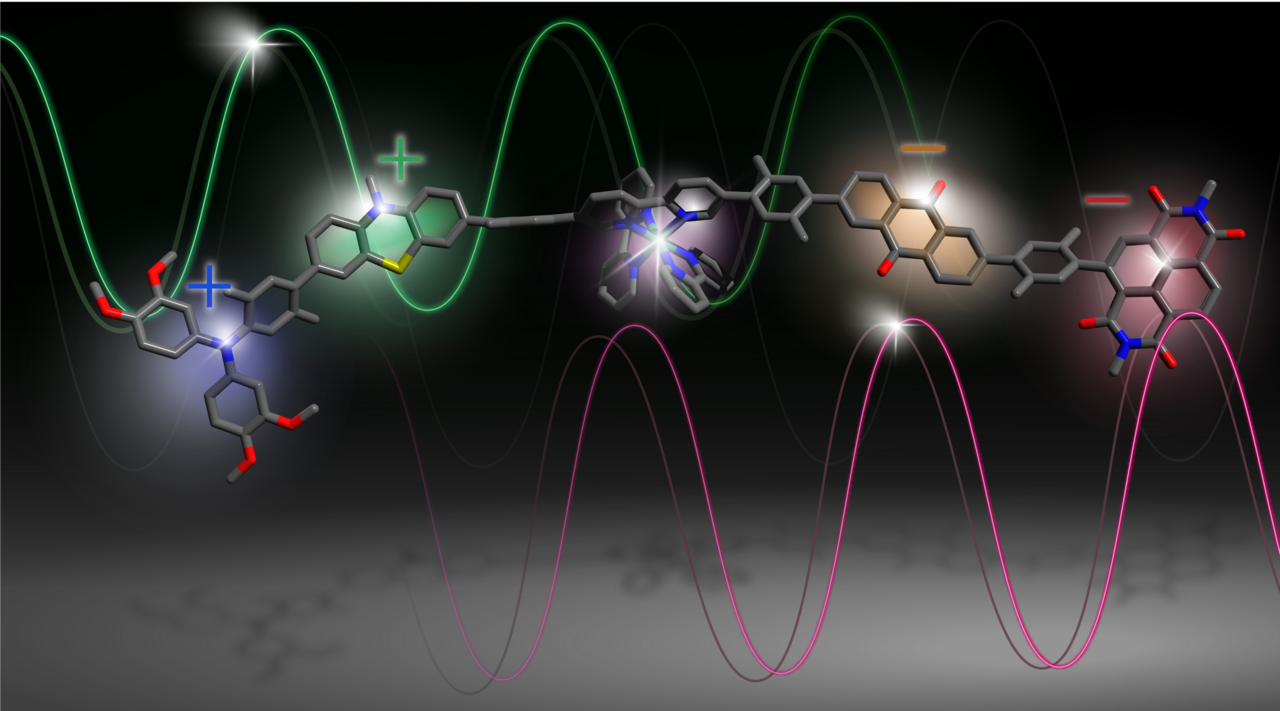
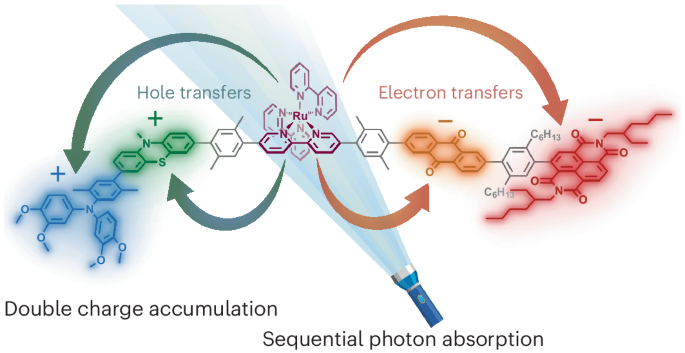
To prevent this destructive photoinduced charge recombination, we have developed a molecule with a built-in redox gradient.5 This allows to transfer the electron to a second electron acceptor and the hole to a second electron donor. The use of this redox gradient enables a fast and unexpectedly efficient separation of the hole and electron over almost 5 nm. This far separation slows down the recombination to the 100 μs regime, providing a useful time window for further (photo)chemistry.
Repeating the photoinduced charge separation
Since the charges are stored far from the photosensitizer, re-excitation of the photosensitizer no longer triggers recombination. Instead, the nearby donor or acceptor performs its intended function. In our experiments we were able to temporarily accumulate charges by sequentially exciting the same photosensitizer. This approach decreases the required photon density by 5–7 orders of magnitude compared to two-chromophore schemes, bringing it towards the range of sunlight. We believe that these fundamental findings can be used in future, more application-oriented work.6
References
1. Hammarström, L. Accumulative Charge Separation for Solar Fuels Production: Coupling Light-Induced Single Electron Transfer to Multielectron Catalysis. Acc. Chem. Res. 48, 840–850 (2015).
2. Kuss-Petermann, M.; Wenger, O. S. Electron Transfer Rate Maxima at Large Donor–Acceptor Distances. J. Am. Chem. Soc. 138, 1349–1358 (2016).
3. Neumann, S.; Kerzig, C.; Wenger, O. S. Quantitative insights into charge-separated states from one- and two-pulse laser experiments relevant for artificial photosynthesis. Chem. Sci. 10, 5624–5633 (2019).
4. Bürgin, T.; Wenger, O. S. Recent Advances and Perspectives in Photodriven Charge Accumulation in Molecular Compounds: A Mini Review. Energy Fuels 35, 18848–18856 (2021).
5. Brändlin, M.; Pfund, B.; Wenger, O. S. Photoinduced double charge accumulation in a molecular compound. Nat. Chem. (2025).
6. Pellegrin, Y.; Odobel, F. Molecular devices featuring sequential photoinduced charge separations for the storage of multiple redox equivalents. Coord. Chem. Rev. 255, 2578–2593 (2011).
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in