Immobilized acyl-transfer molecular reactors enable the solid phase synthesis of sterically hindered peptides
Published in Chemistry, Materials, and Biomedical Research
In the ever-evolving field of peptide therapeutics, the synthesis of complex, sterically hindered peptides remains a formidable challenge. These peptides, which incorporate N-methylated or α,α-disubstituted amino acids, hold immense potential as pharmaceutical agents due to their enhanced stability and membrane permeability. However, conventional solid-phase peptide synthesis (SPPS) methods often struggle with sluggish reaction rates and low yields when faced with these challenging substrates. The groundbreaking research presented in the forthcoming article, "Immobilized acyl-transfer molecular reactors enable the solid phase synthesis of sterically hindered peptides," introduces a transformative solution to this longstanding problem.
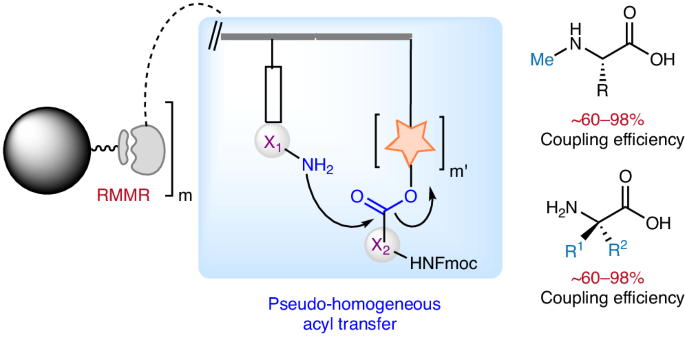
At the heart of this research lies a conceptually novel approach inspired by nature's own peptide synthesis mechanism—the ribosome. We have successfully developed an immobilized ribosome-mimicking molecular reactor (RMMR) that facilitates on-resin proximity-induced intra(inter)reactor acyl transfers. This innovative design bypasses the traditional two-phase acyl transfer mechanism inherent in SPPS, significantly enhancing coupling efficiency for the synthesis of sterically hindered peptides. The RMMR integrates a peptide anchoring point, analogous to peptidyl-tRNA in ribosomes, with multiple carboxyl activating sites, mimicking aminoacyl-tRNA. This configuration enables the rapid formation of amide bonds by bringing reactants into close proximity, thereby circumventing the diffusion limitations that plague conventional SPPS methods. The RMMR's iterative "on/off" recycling mechanism, driven by the generation and subsequent quenching of active esters, dramatically increases the effective molarity of reactive species on the resin surface.
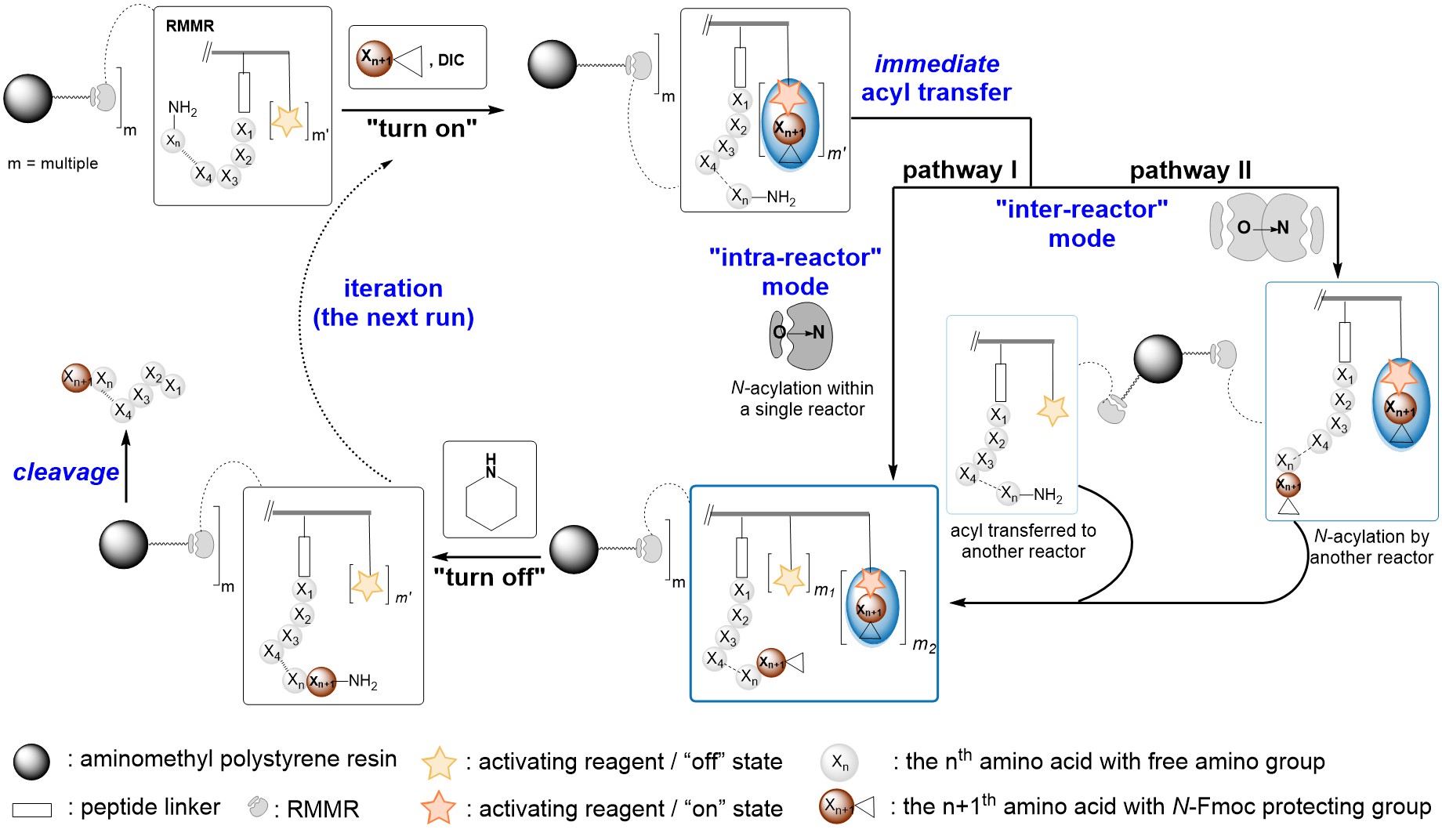
The developed RMMR SPPS technology effectively resolves a pivotal bottleneck encountered in the synthesis of sterically hindered peptides. Traditional solid-phase peptide synthesis (SPPS) methods frequently necessitate the use of substantially excessive reagent quantities and stringent reaction conditions to attain acceptable yields, rendering the process cumbersome and inefficient. In stark contrast, the new protocol based on RMMR technology exhibits remarkable efficiency in coupling N-methylated and α,α-disubstituted amino acids. It achieves crude product purities exceeding 90% for a wide range of peptide sequences, a striking difference from the less than 30% yields typically observed with conventional methods. Moreover, the RMMR protocol demonstrates exceptional efficacy in synthesizing peptide sequences containing multiple consecutive 2-aminoisobutyric acid (Aib) residues (up to six), achieving nearly quantitative crude purities.
The pivotal accomplishments of this research also include the efficient solid-phase synthesis of pharmaceutically important and synthetically challenging peptides cyclosporin A and alamethicin F analogues, which are distinguished by the existence of multiple consecutive N-methylated or α,α-disubstituted amino acids in their sequences. The RMMR SPPS strategy not only surmounts these long-standing challenges but also achieves this feat under exceptionally mild reaction conditions.
The RMMR SPPS utilizes readily available commercial reagents and standard peptide synthesizers, eliminating the need for specialized or complex setups. This seamless compatibility with existing SPPS platforms holds immense promise. It paves the way for the automated, high-throughput synthesis of sterically hindered peptides that are of great pharmaceutical significance, thereby opening up new avenues for drug discovery and development. Looking ahead, the applications of RMMR technology may significantly reshare the community’s capability to study a unique peptide chemical space that was barely accessible in the past. The ability to efficiently synthesize complex, sterically hindered peptides opens new avenues for drug discovery aiming for targeting intracellular receptors or wishing to achieve oral administration of peptide-size molecules. It also enables bio-physical properties studies of such unique chemical spaces such as poly-alpha-methyl peptides, and their application in peptide-based biomaterials or nanomaterials.
In summary, the research presented in "Immobilized acyl-transfer molecular reactors enable the solid phase synthesis of sterically hindered peptides" represents a significant breakthrough in peptide synthesis. The RMMR SPPS has been proven a highly efficient and versatile protocol to overcome the longstanding challenges associated with sterically hindered peptides. This innovative approach not only advances our fundamental understanding of peptide synthesis but also paves the way for the development of novel peptide therapeutics with improved pharmacological profiles. As we stand on the brink of a new era in peptide drug discovery, the RMMR SPPS technology promises to be a driving force behind future scientific and medical advancements. (For details, please read the article at https://doi.org/10.1038/s41557-025-01896-8)
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in